Physical-chemical properties of complex natural fluids
... vapour and melts, as well as subvalence state of metals during transport processes. Based on chemical analyses of gases and condensates from high-temperature fumaroles of the Kudryavy volcano (i Iturup, Kuril Arc, Russia), a thermodynamic simulation of transport and deposition of ore- and rock-formi ...
... vapour and melts, as well as subvalence state of metals during transport processes. Based on chemical analyses of gases and condensates from high-temperature fumaroles of the Kudryavy volcano (i Iturup, Kuril Arc, Russia), a thermodynamic simulation of transport and deposition of ore- and rock-formi ...
Chapter 5 Principles of Chemical Reactivity: Energy and Chemical
... (b) System and Surroundings—The “system” is the reactant(s) and product(s) of a reaction, while the “surroundings” is EVERYTHING else. Suppose we burn gasoline in an internal combustion engine. The gasoline (and air) in the cylinder(s) composes the “system”, while the engine, and the air contacting ...
... (b) System and Surroundings—The “system” is the reactant(s) and product(s) of a reaction, while the “surroundings” is EVERYTHING else. Suppose we burn gasoline in an internal combustion engine. The gasoline (and air) in the cylinder(s) composes the “system”, while the engine, and the air contacting ...
MEDICAL CHEMISTRY STUDY GUIDE
... The chemistry laboratory is not a dangerous place to work as long as all necessary precautions are taken seriously. In the following paragraphs, those important precautions are described. Everyone who works and performs experiments in a laboratory must follow these safety rules at all times. Student ...
... The chemistry laboratory is not a dangerous place to work as long as all necessary precautions are taken seriously. In the following paragraphs, those important precautions are described. Everyone who works and performs experiments in a laboratory must follow these safety rules at all times. Student ...
SCHOOL OF CHEMICAL SCIENCES
... [Bachelor of Science with Honours, B.Sc. (Hons) and Bachelor of Applied Science B.App.Sc.(Hons)] from the School of Chemical Sciences, USM is planned to produce graduates who are knowledgeable, highly skilled and well-mannered and possess excellent work ethics suited for the requirements of the indu ...
... [Bachelor of Science with Honours, B.Sc. (Hons) and Bachelor of Applied Science B.App.Sc.(Hons)] from the School of Chemical Sciences, USM is planned to produce graduates who are knowledgeable, highly skilled and well-mannered and possess excellent work ethics suited for the requirements of the indu ...
Title
... and two PTA bonded by the P atom. The 1H NMR spectrum of 1Cl2 in D2O displays characteristic signals for one PTA (from 3.77 ppm to 4.35 ppm) and one bpy ligand (from 7.29 ppm to 8.73 ppm). The 13C{1H} NMR also confirmed the coordination of the bpy and PTA ligands to the metal. The 31P{1H} NMR displa ...
... and two PTA bonded by the P atom. The 1H NMR spectrum of 1Cl2 in D2O displays characteristic signals for one PTA (from 3.77 ppm to 4.35 ppm) and one bpy ligand (from 7.29 ppm to 8.73 ppm). The 13C{1H} NMR also confirmed the coordination of the bpy and PTA ligands to the metal. The 31P{1H} NMR displa ...
CHAPTER 9 Notes
... theoretical yield: Amount of product one should get based on the chemical equation and the amount of reactants present -One generally calculates this in grams from info given Actual yield: Amount of produce one actually obtains -Generally smaller than the theoretical yield because of impurities and ...
... theoretical yield: Amount of product one should get based on the chemical equation and the amount of reactants present -One generally calculates this in grams from info given Actual yield: Amount of produce one actually obtains -Generally smaller than the theoretical yield because of impurities and ...
Instructor`s Guide to General Chemistry: Guided
... (a) The balanced reaction equation is needed to relate the number of molecules/ions of the reactants to the number of molecules/ions that are produced as products. The number of molecules/ions is measured in units of moles. (b) Steps 2 and 3 make clear what information is given and what needs to be ...
... (a) The balanced reaction equation is needed to relate the number of molecules/ions of the reactants to the number of molecules/ions that are produced as products. The number of molecules/ions is measured in units of moles. (b) Steps 2 and 3 make clear what information is given and what needs to be ...
Teaching with SCIGRESS - Photochemical Dynamics Group
... successfully predicts molecular geometry, neither theory takes into account the relationship between atomic orbitals and electrons in bonds. Valence-bond theory approaches this by describing bonding as the overlap of atomic orbitals from two bonded atoms and explains molecular geometry using hybrid ...
... successfully predicts molecular geometry, neither theory takes into account the relationship between atomic orbitals and electrons in bonds. Valence-bond theory approaches this by describing bonding as the overlap of atomic orbitals from two bonded atoms and explains molecular geometry using hybrid ...
w_4-3 Chemistry of Nitrogen Compounds
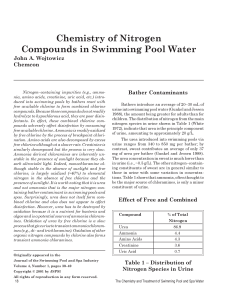
... free available chlorine to form combined chlorine compounds. Because these compounds do not readily hydrolyze to hypochlorous acid, they are poor disinfectants. In effect, these combined chlorine compounds adversely affect disinfection by consuming free available chlorine. Ammonia is readily oxidize ...
... free available chlorine to form combined chlorine compounds. Because these compounds do not readily hydrolyze to hypochlorous acid, they are poor disinfectants. In effect, these combined chlorine compounds adversely affect disinfection by consuming free available chlorine. Ammonia is readily oxidize ...
GCE Chemistry SAMs 2009 onwards pdf
... Give one reason why the value obtained for the enthalpy change in this experiment is lower than the true value. ...
... Give one reason why the value obtained for the enthalpy change in this experiment is lower than the true value. ...
contact - DTU Kemi
... meaning they have two forms which – like the human left and right hand - are mirror images of each other but cannot be superimposed onto each other. The two forms are described by the same chemical formula while in fact being differently structured and potentially with different properties. It is ve ...
... meaning they have two forms which – like the human left and right hand - are mirror images of each other but cannot be superimposed onto each other. The two forms are described by the same chemical formula while in fact being differently structured and potentially with different properties. It is ve ...
THE DIFFUSION MECHANISM OF HYDROCARBONS IN... Jirong Xiao B.S., East China Institute of Chemical Technology
... equilibrium data. The rising trend of diffusivityis also observed for heptane diffusion in 5A where one cage can host two molecules. ...
... equilibrium data. The rising trend of diffusivityis also observed for heptane diffusion in 5A where one cage can host two molecules. ...
Chemistry - Department of Education and Skills
... Department of Education and Science’s Equality of Opportunity Programme. The project developed out of the Department’s scheme of Intervention Projects in Physics and Chemistry which was implemented from 1985 with a view to increasing the participation of girls in the study of the physical sciences. ...
... Department of Education and Science’s Equality of Opportunity Programme. The project developed out of the Department’s scheme of Intervention Projects in Physics and Chemistry which was implemented from 1985 with a view to increasing the participation of girls in the study of the physical sciences. ...
Solutions
... 6.31 a. The heat lost by the metal is equal to the heat gained by the water. Since q = s x m x ∆t, the heat gained by the water is directly proportional to ∆t. Since ∆t is larger for metal A, it lost more heat. Now, each metal has the same mass and ∆t, so the specific heat is directly proportional t ...
... 6.31 a. The heat lost by the metal is equal to the heat gained by the water. Since q = s x m x ∆t, the heat gained by the water is directly proportional to ∆t. Since ∆t is larger for metal A, it lost more heat. Now, each metal has the same mass and ∆t, so the specific heat is directly proportional t ...
HOTS Worksheet
... Q. 1. When a mixture of salicylic acid, acetic anhydride and acetic acid is refluxed, what is the product obtained and what is its use in everyday life ? Ans. Aspirin used as analgesic. Q. 2. Distinguish between a narrow spectrum and broad spectrum antibiotic. Ans. A narrow spectrum antibiotic work ...
... Q. 1. When a mixture of salicylic acid, acetic anhydride and acetic acid is refluxed, what is the product obtained and what is its use in everyday life ? Ans. Aspirin used as analgesic. Q. 2. Distinguish between a narrow spectrum and broad spectrum antibiotic. Ans. A narrow spectrum antibiotic work ...
Chapter One Hemilabile Ligands in Transition
... functional groups to the phosphorus atom.9,10,11 Such complexes have been used in a range of catalytic reactions due to the hemilabile ligand being able to furnish open coordination sites and stabilize reactive transition metal centers during the course of a reaction.12 This reversible protection of ...
... functional groups to the phosphorus atom.9,10,11 Such complexes have been used in a range of catalytic reactions due to the hemilabile ligand being able to furnish open coordination sites and stabilize reactive transition metal centers during the course of a reaction.12 This reversible protection of ...
Transition state theory
Transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes.TST is used primarily to understand qualitatively how chemical reactions take place. TST has been less successful in its original goal of calculating absolute reaction rate constants because the calculation of absolute reaction rates requires precise knowledge of potential energy surfaces, but it has been successful in calculating the standard enthalpy of activation (Δ‡Hɵ), the standard entropy of activation (Δ‡Sɵ), and the standard Gibbs energy of activation (Δ‡Gɵ) for a particular reaction if its rate constant has been experimentally determined. (The ‡ notation refers to the value of interest at the transition state.)This theory was developed simultaneously in 1935 by Henry Eyring, then at Princeton University, and by Meredith Gwynne Evans and Michael Polanyi of the University of Manchester. TST is also referred to as ""activated-complex theory,"" ""absolute-rate theory,"" and ""theory of absolute reaction rates.""Before the development of TST, the Arrhenius rate law was widely used to determine energies for the reaction barrier. The Arrhenius equation derives from empirical observations and ignores any mechanistic considerations, such as whether one or more reactive intermediates are involved in the conversion of a reactant to a product. Therefore, further development was necessary to understand the two parameters associated with this law, the pre-exponential factor (A) and the activation energy (Ea). TST, which led to the Eyring equation, successfully addresses these two issues; however, 46 years elapsed between the publication of the Arrhenius rate law, in 1889, and the Eyring equation derived from TST, in 1935. During that period, many scientists and researchers contributed significantly to the development of the theory.