PHOSPHORUS AND SULFUR COSMOCHEMISTRY
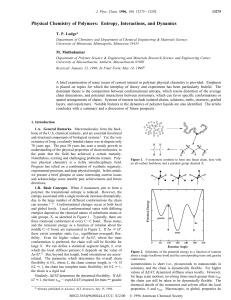
... phosphorus compounds, energetic organic compounds, or unusual physical conditions. Meteoritic schreibersite provided an abundant source of reactive phosphorus for the early Earth. Water corrodes schreibersite to form a mixed valence series of phosphorus compounds. Schreibersite corrosion was studied ...
... phosphorus compounds, energetic organic compounds, or unusual physical conditions. Meteoritic schreibersite provided an abundant source of reactive phosphorus for the early Earth. Water corrodes schreibersite to form a mixed valence series of phosphorus compounds. Schreibersite corrosion was studied ...
Acid Base Equilibrium Diploma Questions
... The temperature of the solution is constant. No solid is present at the bottom of the container. Vigorous stirring does not dissolve more of the solid. If the solution is heated, the amount of the solid that dissolves changes. Use the following information to answer the next 2 questions. Sodium azid ...
... The temperature of the solution is constant. No solid is present at the bottom of the container. Vigorous stirring does not dissolve more of the solid. If the solution is heated, the amount of the solid that dissolves changes. Use the following information to answer the next 2 questions. Sodium azid ...
2 - Montville.net
... The mole enables chemists to move from the microscopic world of atoms and molecules to the real world of grams . Stoichiometry problems are classified between the information given in the problem and the information you are expected to find, the unknown. The given and the unknown may be expressed i ...
... The mole enables chemists to move from the microscopic world of atoms and molecules to the real world of grams . Stoichiometry problems are classified between the information given in the problem and the information you are expected to find, the unknown. The given and the unknown may be expressed i ...
Arsenic behaviour in subsurface hydrogeochemical systems
... is done and the value -12.13 for log K2 is recommended (Table 1). The arsenic species AsO+ and As033- doesn't present great interest for natural ground and surface waters because their stability field is only at pHc0.3 or >13.4 respectively (Sergeeva and Khodakovskiy, 1969). However, for some specia ...
... is done and the value -12.13 for log K2 is recommended (Table 1). The arsenic species AsO+ and As033- doesn't present great interest for natural ground and surface waters because their stability field is only at pHc0.3 or >13.4 respectively (Sergeeva and Khodakovskiy, 1969). However, for some specia ...
CHAPTER 15 ACIDS AND BASES
... Step 1: Express the equilibrium concentrations of all species in terms of initial concentrations and a single unknown x, that represents the change in concentration. Let (−x) be the depletion in concentration (mol/L) of HF. From the stoichiometry of the reaction, it follows that the increase in conc ...
... Step 1: Express the equilibrium concentrations of all species in terms of initial concentrations and a single unknown x, that represents the change in concentration. Let (−x) be the depletion in concentration (mol/L) of HF. From the stoichiometry of the reaction, it follows that the increase in conc ...
Coordination Chemistry Reviews Iron–dinitrogen coordination
... into molecules such as proteins and nuclei acids, reduced forms of dinitrogen are needed for biomolecule synthesis. Although having a source of reduced N2 is crucial for life to exist, the process of reducing N2 is very energy intensive. The inertness of the dinitrogen molecule is partly due to its ...
... into molecules such as proteins and nuclei acids, reduced forms of dinitrogen are needed for biomolecule synthesis. Although having a source of reduced N2 is crucial for life to exist, the process of reducing N2 is very energy intensive. The inertness of the dinitrogen molecule is partly due to its ...
National German Competition
... 4. The radiation of 1 is executed in a closed vessel. After the reaction there is an overpressure within the vessel, a gas was generated. 5. The reaction was followed by infrared spectroscopy. A sample of compound 1 gave a certain spectrum (upper part of the image). Then the sample was radiated with ...
... 4. The radiation of 1 is executed in a closed vessel. After the reaction there is an overpressure within the vessel, a gas was generated. 5. The reaction was followed by infrared spectroscopy. A sample of compound 1 gave a certain spectrum (upper part of the image). Then the sample was radiated with ...
Isopropanol oxidation by pure metal oxide
... reduction of the metal oxide surface can usually take place. Other methods involve the adsorption of CO2 and NH3 . CO2 and NH3 do not measure all the surface sites. CO2 only adsorbs on basic OH groups on the surface and NH3 only adsorbs on Lewis and Bronsted acid sites. Furthermore, the above method ...
... reduction of the metal oxide surface can usually take place. Other methods involve the adsorption of CO2 and NH3 . CO2 and NH3 do not measure all the surface sites. CO2 only adsorbs on basic OH groups on the surface and NH3 only adsorbs on Lewis and Bronsted acid sites. Furthermore, the above method ...
Chapter 4
... This equation says that all sodium chloride that enters the solution ends up as Na1 and Cl2 ions; there are no undissociated NaCl units in solution. Table 4.1 lists examples of strong electrolytes, weak electrolytes, and nonelectrolytes. Ionic compounds, such as sodium chloride, potassium iodide (KI ...
... This equation says that all sodium chloride that enters the solution ends up as Na1 and Cl2 ions; there are no undissociated NaCl units in solution. Table 4.1 lists examples of strong electrolytes, weak electrolytes, and nonelectrolytes. Ionic compounds, such as sodium chloride, potassium iodide (KI ...
Chapter 3 Stoichiometry: Calculations with Chemical
... • One mole of atoms, ions, or molecules contains Avogadro’s number of those particles • One mole of molecules or formula units contains Avogadro’s number times the number of atoms or ions of each element in the compound Stoichiometry ...
... • One mole of atoms, ions, or molecules contains Avogadro’s number of those particles • One mole of molecules or formula units contains Avogadro’s number times the number of atoms or ions of each element in the compound Stoichiometry ...
Chapter 3 Stoichiometry: Calculations with Chemical Formulas and
... • One mole of atoms, ions, or molecules contains Avogadro s number of those particles • One mole of molecules or formula units contains Avogadro s number times the number of atoms or ions of each element in the compound Stoichiometry ...
... • One mole of atoms, ions, or molecules contains Avogadro s number of those particles • One mole of molecules or formula units contains Avogadro s number times the number of atoms or ions of each element in the compound Stoichiometry ...
General and Inorganic Chemistry – Laboratory Techniques
... Knowledge of students on Chemistry at the beginning of their graduate studies is rather different. Most of the students do not have proper laboratory expertise. This educational experience prompted the faculty of the institute to compile an educational material that can help students to make themsel ...
... Knowledge of students on Chemistry at the beginning of their graduate studies is rather different. Most of the students do not have proper laboratory expertise. This educational experience prompted the faculty of the institute to compile an educational material that can help students to make themsel ...
The Mole
... Makes 3 dozen If we had the specified amount of all ingredients listed, could we make 4 dozen cookies? What if we had 6 eggs and twice as much of everything else, could we make 9 dozen cookies? What if we only had one egg, could we make 3 dozen cookies? ...
... Makes 3 dozen If we had the specified amount of all ingredients listed, could we make 4 dozen cookies? What if we had 6 eggs and twice as much of everything else, could we make 9 dozen cookies? What if we only had one egg, could we make 3 dozen cookies? ...
Transition state theory
Transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes.TST is used primarily to understand qualitatively how chemical reactions take place. TST has been less successful in its original goal of calculating absolute reaction rate constants because the calculation of absolute reaction rates requires precise knowledge of potential energy surfaces, but it has been successful in calculating the standard enthalpy of activation (Δ‡Hɵ), the standard entropy of activation (Δ‡Sɵ), and the standard Gibbs energy of activation (Δ‡Gɵ) for a particular reaction if its rate constant has been experimentally determined. (The ‡ notation refers to the value of interest at the transition state.)This theory was developed simultaneously in 1935 by Henry Eyring, then at Princeton University, and by Meredith Gwynne Evans and Michael Polanyi of the University of Manchester. TST is also referred to as ""activated-complex theory,"" ""absolute-rate theory,"" and ""theory of absolute reaction rates.""Before the development of TST, the Arrhenius rate law was widely used to determine energies for the reaction barrier. The Arrhenius equation derives from empirical observations and ignores any mechanistic considerations, such as whether one or more reactive intermediates are involved in the conversion of a reactant to a product. Therefore, further development was necessary to understand the two parameters associated with this law, the pre-exponential factor (A) and the activation energy (Ea). TST, which led to the Eyring equation, successfully addresses these two issues; however, 46 years elapsed between the publication of the Arrhenius rate law, in 1889, and the Eyring equation derived from TST, in 1935. During that period, many scientists and researchers contributed significantly to the development of the theory.