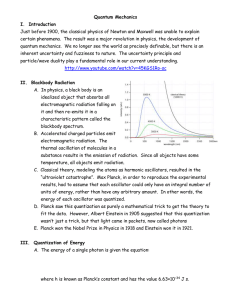
Objective 6: TSW explain how the quantum
... demonstrates that particles have wave behaviors • If you are dealing with an electron moving at speeds near the speed of light you find it has a sufficiently large wavelength so that its wavelength is close to that of ordinary electromagnetic radiation • In the late 1920s, scientists observed electr ...
... demonstrates that particles have wave behaviors • If you are dealing with an electron moving at speeds near the speed of light you find it has a sufficiently large wavelength so that its wavelength is close to that of ordinary electromagnetic radiation • In the late 1920s, scientists observed electr ...
Chapter 7 Name Atomic Structure and Periodicity Any day you don`t
... Curved (accelerated) motion means that electron should do what? = ASSUMPTIONS BASED IN CLASSICAL PHYSICS!!! Quantum physics: 1. Angular motion of electron (mass, velocity, and orbital radius) occurs at certain increments 2. Only certain electron energies are allowed in the hydrogen atom ...
... Curved (accelerated) motion means that electron should do what? = ASSUMPTIONS BASED IN CLASSICAL PHYSICS!!! Quantum physics: 1. Angular motion of electron (mass, velocity, and orbital radius) occurs at certain increments 2. Only certain electron energies are allowed in the hydrogen atom ...
Chapter 4
... Particle Nature of Light Bohr Model of the H atom 1913 – Danish physicist – Niels Bohr Single e- circled around nucleus in allowed paths or orbits e- has fixed E when in this orbit (lowest E closest to nucleus) Lot of empty space between nucleus and e- in which e- cannot be in E increas ...
... Particle Nature of Light Bohr Model of the H atom 1913 – Danish physicist – Niels Bohr Single e- circled around nucleus in allowed paths or orbits e- has fixed E when in this orbit (lowest E closest to nucleus) Lot of empty space between nucleus and e- in which e- cannot be in E increas ...
Arrangement of Electrons in Atoms
... a particle and a wave. What about electrons? Louis De Broglie stated that electrons could be considered waves confined to a space around an atomic nucleus. Electron waves can exist, but only at specific frequencies corresponding to specific ...
... a particle and a wave. What about electrons? Louis De Broglie stated that electrons could be considered waves confined to a space around an atomic nucleus. Electron waves can exist, but only at specific frequencies corresponding to specific ...
STATE UNIVERSITY OF NEW YORK COLLEGE OF TECHNOLOGY CANTON, NEW YORK
... E. Show how electrons bind atoms together in metals and covalent solids. F. Calculate the electronic specific heat and, using the idea of a relaxation time, calculate the thermal conductivity due to free electrons, and discuss electrical current, resistivity, the Wiedemann-Franz law and the Hall ...
... E. Show how electrons bind atoms together in metals and covalent solids. F. Calculate the electronic specific heat and, using the idea of a relaxation time, calculate the thermal conductivity due to free electrons, and discuss electrical current, resistivity, the Wiedemann-Franz law and the Hall ...
In 1913 Bohr proposed his quantized shell model of the atom to
... move in orbits of fixed size and energy. The energy of an electron depends on the size of the orbit and is lower for smaller orbits. Radiation can occur only when the electron jumps from one orbit to another. The atom will be completely stable in the state with the smallest orbit, since there is no ...
... move in orbits of fixed size and energy. The energy of an electron depends on the size of the orbit and is lower for smaller orbits. Radiation can occur only when the electron jumps from one orbit to another. The atom will be completely stable in the state with the smallest orbit, since there is no ...
Fermi-Dirac Statistics
... • E is the energy we are interested in. • T is the temperature at equilibrium. Fermi-Dirac function at Zero Temperature • Plot the function on the board in the limit of T→0. Show the step-wise nature of the transition when E=ΕF. • Note how the step-wise transition at T=0 occurs because E-EF is divid ...
... • E is the energy we are interested in. • T is the temperature at equilibrium. Fermi-Dirac function at Zero Temperature • Plot the function on the board in the limit of T→0. Show the step-wise nature of the transition when E=ΕF. • Note how the step-wise transition at T=0 occurs because E-EF is divid ...
Matter: a Material World
... Energy Levels in Atoms Electrons in atoms do NOT “orbit” around the nucleus like little planets - their position better described by probability waves However, they do move in different “energy states” – some electrons in a given atom have more energy than others These energy states are “quantized” ...
... Energy Levels in Atoms Electrons in atoms do NOT “orbit” around the nucleus like little planets - their position better described by probability waves However, they do move in different “energy states” – some electrons in a given atom have more energy than others These energy states are “quantized” ...
Bohr`s Model of the Atom - Mr. Walsh`s AP Chemistry
... The Bohr Model of the Hydrogen Atom The Bohr model worked well for hydrogen. However, the equations could not be solved exactly for atoms with more than one electron, because of the additional effects that electrons exert on each other (Coulomb force kq q F d12 2 ). By the mid-1920s, quantum physi ...
... The Bohr Model of the Hydrogen Atom The Bohr model worked well for hydrogen. However, the equations could not be solved exactly for atoms with more than one electron, because of the additional effects that electrons exert on each other (Coulomb force kq q F d12 2 ). By the mid-1920s, quantum physi ...
Chapter 5: Electrons in Atoms
... Example: cars (have wavelengths to small to be seen even with sensitive equipment) ...
... Example: cars (have wavelengths to small to be seen even with sensitive equipment) ...
Density Matrix
... state variables are microscopic, and a state is specified by giving the positions and velocities of all particles as a function of time. Obviously, this does not generalize to the quantum theory very easily, thus in our work we will try to adhere as closely as possible to the first meaning, but it n ...
... state variables are microscopic, and a state is specified by giving the positions and velocities of all particles as a function of time. Obviously, this does not generalize to the quantum theory very easily, thus in our work we will try to adhere as closely as possible to the first meaning, but it n ...
electron scattering (2)
... But the electron interacts with charge elements dQ inside the nucleus: ...
... But the electron interacts with charge elements dQ inside the nucleus: ...
powerpoint - University of Illinois Urbana
... National Science Foundation under Grant CHE-1118616 (CAREER), and the Camille & Henry Dreyfus Foundation, Inc. through the Camille Dreyfus Teacher-Scholar program. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily ...
... National Science Foundation under Grant CHE-1118616 (CAREER), and the Camille & Henry Dreyfus Foundation, Inc. through the Camille Dreyfus Teacher-Scholar program. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily ...
File
... electron is likely to be located. Principal (n), 1-7, gives the energy level Sublevel (l), s-p-d-f, gives the shape of region Orbital (m), gives the orientation in space of the shapes Spin (s), clockwise or counterclockwise ...
... electron is likely to be located. Principal (n), 1-7, gives the energy level Sublevel (l), s-p-d-f, gives the shape of region Orbital (m), gives the orientation in space of the shapes Spin (s), clockwise or counterclockwise ...
Chapter2. Elements of quantum mechanics
... a. A projectile electron of kinetic energy 12.2 eV collides with a hydrogen atom in a gas discharge tube. Find the n-th energy level to which the electron in the hydrogen atom gets excited. b. Calculate the possible wavelengths of radiation (in nm) that will be emitted from the excited H atom in par ...
... a. A projectile electron of kinetic energy 12.2 eV collides with a hydrogen atom in a gas discharge tube. Find the n-th energy level to which the electron in the hydrogen atom gets excited. b. Calculate the possible wavelengths of radiation (in nm) that will be emitted from the excited H atom in par ...