Lecture 33 - Stimulated Absorption
... ii. Stimulated absorption occurs when a photon strikes an atom with just exactly the proper energy to induce an electronic transition between two energy states. iii. Einstein’s contribution was to show that there was a third process, stimulated emission, which could cause a downward electronic trans ...
... ii. Stimulated absorption occurs when a photon strikes an atom with just exactly the proper energy to induce an electronic transition between two energy states. iii. Einstein’s contribution was to show that there was a third process, stimulated emission, which could cause a downward electronic trans ...
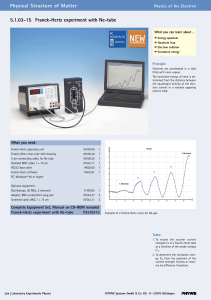
5.1.03-15 Franck-Hertz experiment with Ne
... 1913: An isolated atom consists of a positively charged nucleus about which electrons are distributed in successive orbits. He also postulated that only those orbits occur for which the angular momentum of the electron is an integral multiple of h/2p, i.e. n*h/2p, where n is an integer and h is Plan ...
... 1913: An isolated atom consists of a positively charged nucleus about which electrons are distributed in successive orbits. He also postulated that only those orbits occur for which the angular momentum of the electron is an integral multiple of h/2p, i.e. n*h/2p, where n is an integer and h is Plan ...
By: 3rd Period Chemistry Actinide Ionization Energy Probability
... Shows interactions between light and electrons occur at specific energies emission ...
... Shows interactions between light and electrons occur at specific energies emission ...
PHYS 221: Homework Assignment 3 This homework due just prior
... b) [2 points] Now suppose that the electron is replaced by a photon having the same wavelength as the electron had. Will it in general be Bragg reflected or not? If not, why not? c) [2 points] Now suppose that the electron is replaced by a photon having the same momentum as the electron had. Will it ...
... b) [2 points] Now suppose that the electron is replaced by a photon having the same wavelength as the electron had. Will it in general be Bragg reflected or not? If not, why not? c) [2 points] Now suppose that the electron is replaced by a photon having the same momentum as the electron had. Will it ...
sch4u-quantumtheory
... Quantum mechanics, or wave mechanics, is the treatment of atomic structure through the wavelike properties of the electron Erwin Schrödinger developed an equation to describe the hydrogen atom A wave function is a solution to the Schrödinger equation and represents an energy state of the atom ...
... Quantum mechanics, or wave mechanics, is the treatment of atomic structure through the wavelike properties of the electron Erwin Schrödinger developed an equation to describe the hydrogen atom A wave function is a solution to the Schrödinger equation and represents an energy state of the atom ...
Claude Cohen-Tannoudji Scott Lectures Cambridge, March 9 2011
... corresponding to 2 possible paths which can be followed by atoms Can we calculate the phase shift between the 2 wave functions due to various causes (free propagation, laser, external or inertial fields)? The 2 possible paths are represented in the figure above by lines which suggest trajectories of ...
... corresponding to 2 possible paths which can be followed by atoms Can we calculate the phase shift between the 2 wave functions due to various causes (free propagation, laser, external or inertial fields)? The 2 possible paths are represented in the figure above by lines which suggest trajectories of ...
CMC Chapter 05
... The Wave Nature of Light (cont.) • The wavelength (λ) is the shortest distance between equivalent points on a continuous wave. • The frequency (ν) is the number of waves that pass a given point per second. • The amplitude is the wave’s height from the origin to a crest. ...
... The Wave Nature of Light (cont.) • The wavelength (λ) is the shortest distance between equivalent points on a continuous wave. • The frequency (ν) is the number of waves that pass a given point per second. • The amplitude is the wave’s height from the origin to a crest. ...
C. - Elliott County Schools
... • The arrangement of electrons in an atom is called the atom’s electron configuration. • Electron configurations are defined by the aufbau principle, the Pauli exclusion principle, and Hund’s rule. • An element’s valence electrons determine the chemical properties of the element. • Electron conf ...
... • The arrangement of electrons in an atom is called the atom’s electron configuration. • Electron configurations are defined by the aufbau principle, the Pauli exclusion principle, and Hund’s rule. • An element’s valence electrons determine the chemical properties of the element. • Electron conf ...
Chemistry: Matter and Change
... Bohr's Model of the Atom (cont.) • Bohr’s model explained the hydrogen’s spectral lines, but failed to explain any other element’s lines. • The behavior of electrons is still not fully understood, but it is known they do not move around the nucleus in circular orbits. ...
... Bohr's Model of the Atom (cont.) • Bohr’s model explained the hydrogen’s spectral lines, but failed to explain any other element’s lines. • The behavior of electrons is still not fully understood, but it is known they do not move around the nucleus in circular orbits. ...
Gen Chem Ch 5 notes
... The Wave Nature of Light (cont.) • The wavelength (λ) is the shortest distance between equivalent points on a continuous wave. • The frequency (ν) is the number of waves that pass a given point per second. • The amplitude is the wave’s height from the origin to a crest. ...
... The Wave Nature of Light (cont.) • The wavelength (λ) is the shortest distance between equivalent points on a continuous wave. • The frequency (ν) is the number of waves that pass a given point per second. • The amplitude is the wave’s height from the origin to a crest. ...
Document
... Belief: Attractive force between the positively charged nucleus and an electron orbiting around is equal to the centrifugal exerted on the electron. This balance determines the electron’s radius. Challenge: A force is exerted on the electron, then, the electron should accelerate continuously accordi ...
... Belief: Attractive force between the positively charged nucleus and an electron orbiting around is equal to the centrifugal exerted on the electron. This balance determines the electron’s radius. Challenge: A force is exerted on the electron, then, the electron should accelerate continuously accordi ...
Electron Configurations
... Principle we can not know the exact position and motion of electrons with complete certainty. • We can only describe the probable locations of electrons. • We will describe the location of electrons when the atom is at its lowest energy . • These are called “ground state” configurations. • If electr ...
... Principle we can not know the exact position and motion of electrons with complete certainty. • We can only describe the probable locations of electrons. • We will describe the location of electrons when the atom is at its lowest energy . • These are called “ground state” configurations. • If electr ...
Quantum number
... Use arrows to write the electron configuration for an atom of an element whose atomic number is 8. Atomic Number = # of protons # protons = # electrons = 8 Use the orbital filling chart to place the 8 electrons in their proper orbitals. Remember, the s orbital can only hold 2 electrons and the ...
... Use arrows to write the electron configuration for an atom of an element whose atomic number is 8. Atomic Number = # of protons # protons = # electrons = 8 Use the orbital filling chart to place the 8 electrons in their proper orbitals. Remember, the s orbital can only hold 2 electrons and the ...