Chapter 5
... the spin quantum number (ms). E. none of these choices is correct 21. Atomic orbitals developed using quantum mechanics A. describe regions of space in which one is most likely to find an electron. B. describe exact paths for electron motion. C. give a description of the atomic structure which is es ...
... the spin quantum number (ms). E. none of these choices is correct 21. Atomic orbitals developed using quantum mechanics A. describe regions of space in which one is most likely to find an electron. B. describe exact paths for electron motion. C. give a description of the atomic structure which is es ...
Do your homework on a separate piece of paper, or
... 33. How is Schrödinger’s wave equation similar to the electron in the box equation you derived in 31)? How is it different? “electron in a box” EK = n2h2/[8mL2], Schrödinger’s EK = n2h2/[8m2]. 34. How do the electron orbitals in the Schrödinger model differ from those in the Bohr model? Schrödinger ...
... 33. How is Schrödinger’s wave equation similar to the electron in the box equation you derived in 31)? How is it different? “electron in a box” EK = n2h2/[8mL2], Schrödinger’s EK = n2h2/[8m2]. 34. How do the electron orbitals in the Schrödinger model differ from those in the Bohr model? Schrödinger ...
Advanced Chemistry - Forestville Middle
... for scientists to describe the electrons in an atom in terms of wave properties Schrödinger developed an equation to incorporate both the wave and particle properties of the electron. The square of a wave function is the probability density, or the probability that an electron will be found at a giv ...
... for scientists to describe the electrons in an atom in terms of wave properties Schrödinger developed an equation to incorporate both the wave and particle properties of the electron. The square of a wave function is the probability density, or the probability that an electron will be found at a giv ...
Calculation of the Fermi wave vector for thin films, T. B
... dimensionless electronic density ρ is 0.5. The choice of copper is connected to the view that the Fermi surface for this metal is quite similar to a spherical surface [9], therefore our model is more realistic than for other metals. The results of calculation of the Fermi wave vector kF are shown in ...
... dimensionless electronic density ρ is 0.5. The choice of copper is connected to the view that the Fermi surface for this metal is quite similar to a spherical surface [9], therefore our model is more realistic than for other metals. The results of calculation of the Fermi wave vector kF are shown in ...
PHYS-2020: General Physics II Problem Set 3, Spring 2017
... 8. At rest, a car’s horn sounds the note A (440 Hz). The horn is sounded while the car is moving down the street. A bicyclist moving in the same direction with one-third the car’s speed hears a frequency of 415 Hz. (a) Is the cyclist ahead or behind the car? (b) What is the speed of the car? 9. Two ...
... 8. At rest, a car’s horn sounds the note A (440 Hz). The horn is sounded while the car is moving down the street. A bicyclist moving in the same direction with one-third the car’s speed hears a frequency of 415 Hz. (a) Is the cyclist ahead or behind the car? (b) What is the speed of the car? 9. Two ...
Disordered Structures Lecture 1, Part 1
... with chemical (covalent) binding • Random network of bonds • Number of bonds per atom preserved – no unsatisfied bonds • Distribution of bond lengths, bond angles, ring sizes. • Adjacent structural units may be rotated relative to one ...
... with chemical (covalent) binding • Random network of bonds • Number of bonds per atom preserved – no unsatisfied bonds • Distribution of bond lengths, bond angles, ring sizes. • Adjacent structural units may be rotated relative to one ...
physical chemistry ii chem 3354
... value of k the energy of this particle is NOT quantized. – k controls the frequency of the cosine wave. • Large k high momentum many oscillations wiggly wavefunction ...
... value of k the energy of this particle is NOT quantized. – k controls the frequency of the cosine wave. • Large k high momentum many oscillations wiggly wavefunction ...
Quantum Mechanics
... Although we cannot precisely define an electron’s orbit, we can obtain the probability of finding an electron at a given point around the nucleus. – Erwin Schrodinger defined this probability in a mathematical expression called a wave function, denoted y (psi). – The probability of finding a particl ...
... Although we cannot precisely define an electron’s orbit, we can obtain the probability of finding an electron at a given point around the nucleus. – Erwin Schrodinger defined this probability in a mathematical expression called a wave function, denoted y (psi). – The probability of finding a particl ...
Atoms
... You have learned that a light beam consists of photons. The intensity of a beam is proportional to the number of photons. The distribution of intensity versus frequency (or wavelength) is a spectrum. A white light consists of photons with all frequencies in the visible region, and it has a continuou ...
... You have learned that a light beam consists of photons. The intensity of a beam is proportional to the number of photons. The distribution of intensity versus frequency (or wavelength) is a spectrum. A white light consists of photons with all frequencies in the visible region, and it has a continuou ...
Atomic Structure Lecture 6 - Introduction Lecture 6
... The Wave-Particle Duality of Matter and Energy • It turns out both light energy and particles have both wavelike and particle-like behaviors. ...
... The Wave-Particle Duality of Matter and Energy • It turns out both light energy and particles have both wavelike and particle-like behaviors. ...
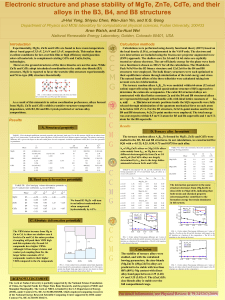
Electronic structure and phase stability of MgTe, ZnTe, CdTe, and
... Calculations were performed using density functional theory (DFT) based on the local density (LDA), as implemented in the VASP code. The electron and core interactions are included using the frozen-core projector augmented wave (PAW) approach. The shallow-core Zn 3d and Cd 4d states are explicitly t ...
... Calculations were performed using density functional theory (DFT) based on the local density (LDA), as implemented in the VASP code. The electron and core interactions are included using the frozen-core projector augmented wave (PAW) approach. The shallow-core Zn 3d and Cd 4d states are explicitly t ...
atomic history
... Using alpha particles, discovered a neutral atomic particle with a mass close to that of the proton. This is what we know as a neutron. ...
... Using alpha particles, discovered a neutral atomic particle with a mass close to that of the proton. This is what we know as a neutron. ...
Physics 200 Class #1 Outline
... Bohr comes to a line of reasoning known as the Copenhagen Interpretation of Quantum Theory. The wave function describes all of the possible outcomes of an experiment. In a measurement, one of these possibilities becomes known. When does this collapse of the wave function to the measured position tak ...
... Bohr comes to a line of reasoning known as the Copenhagen Interpretation of Quantum Theory. The wave function describes all of the possible outcomes of an experiment. In a measurement, one of these possibilities becomes known. When does this collapse of the wave function to the measured position tak ...
ELECTRIC AND MAGNETIC PROPERTIES OF A
... In this paper we develop the appropriate technique, we determine the quasiparticle dispersion law, and we construct the kinetic equations. In the particular case of zero temperature and absence of recombination, we obtain the stationary state of the system and calculate the interband absorption coef ...
... In this paper we develop the appropriate technique, we determine the quasiparticle dispersion law, and we construct the kinetic equations. In the particular case of zero temperature and absence of recombination, we obtain the stationary state of the system and calculate the interband absorption coef ...
Orbitals
... arrangement in terms of which energy levels and sublevels are occupied Uses the building-up principal Hund’s Rule: When electrons are placed in a set of orbitals of equal energy, the orbitals will be occupied by one electron each before pairing together ...
... arrangement in terms of which energy levels and sublevels are occupied Uses the building-up principal Hund’s Rule: When electrons are placed in a set of orbitals of equal energy, the orbitals will be occupied by one electron each before pairing together ...
Particle in the box
... the edges of the box yields spatial boundary conditions. We seek then solutions of the time-dependent Schrödinger equation: ...
... the edges of the box yields spatial boundary conditions. We seek then solutions of the time-dependent Schrödinger equation: ...
Atomic Structure, Eelectronic Bonding, Periodicity, orbitals
... • Practically speaking atoms that have been discovered or made up to this point in time only have electrons in s, p, d, or f orbitals in their ground state configurations. • Each wave function with an allowed combination of n, l, and ml values describes an atomic orbital, a particular spatial distri ...
... • Practically speaking atoms that have been discovered or made up to this point in time only have electrons in s, p, d, or f orbitals in their ground state configurations. • Each wave function with an allowed combination of n, l, and ml values describes an atomic orbital, a particular spatial distri ...
JCE0597 p605 Numerical Methods for Finding Momentum Space
... the momentum representation of the wave function and demonstrated how to transform the spatial wave function for the particle in the box into momentum space using analytical methods. Prior to this work the subject of the momentum wave function for the particle in the box was given a brief treatment ...
... the momentum representation of the wave function and demonstrated how to transform the spatial wave function for the particle in the box into momentum space using analytical methods. Prior to this work the subject of the momentum wave function for the particle in the box was given a brief treatment ...
ELECTRON I: Free electron model
... explanation. For example, the existence of a ‘bandgap’ for semiconductors. We will then show that a periodic potential will introduce energy bands, and possibly a bandgap. And the solution (wave function of electrons) for a lattice will be always of a ‘Bloch function’ form. Once the wave functions a ...
... explanation. For example, the existence of a ‘bandgap’ for semiconductors. We will then show that a periodic potential will introduce energy bands, and possibly a bandgap. And the solution (wave function of electrons) for a lattice will be always of a ‘Bloch function’ form. Once the wave functions a ...
Quantum Readiness
... Using good instrumentation, the researcher makes a careful study of the location of the particle in this well. The position is measured many times, and after each measurement the particle is once again allowed to reach thermal equilibrium near absolute zero, that is return to its ground state. Based ...
... Using good instrumentation, the researcher makes a careful study of the location of the particle in this well. The position is measured many times, and after each measurement the particle is once again allowed to reach thermal equilibrium near absolute zero, that is return to its ground state. Based ...