Heat and its Transfer Study Guide
... Heat from the water moves to the two spoons. But one spoon feels hotter. Why is one spoon hotter than the other? If you touched the metal spoon it would feel very hot. But the wooden spoon would not feel hot at all. That is because metal is a conductor of heat. The wooden spoon is not. Conductors of ...
... Heat from the water moves to the two spoons. But one spoon feels hotter. Why is one spoon hotter than the other? If you touched the metal spoon it would feel very hot. But the wooden spoon would not feel hot at all. That is because metal is a conductor of heat. The wooden spoon is not. Conductors of ...
Lecture 2 Intro to Heat Flow
... (1 day ≈ 80,000 s) surface area: 2 m x 1 m = 2 m2 50 W/m2 ! — or one lightbulb Types of Heat Transport conduction convection radiation—electromagnetic radiation advection Relationship Between Heat Flow & T Gradient: Fourier’s Law The rate of heat flow is proportional to the difference in heat betwee ...
... (1 day ≈ 80,000 s) surface area: 2 m x 1 m = 2 m2 50 W/m2 ! — or one lightbulb Types of Heat Transport conduction convection radiation—electromagnetic radiation advection Relationship Between Heat Flow & T Gradient: Fourier’s Law The rate of heat flow is proportional to the difference in heat betwee ...
Thermodynamics
... Heat is a form of energy so we can always use Joules. More common in thermodynamics is the calorie: By definition 1 calorie is the amount of heat required to change the temperature of 1 gram of water 1°C. ...
... Heat is a form of energy so we can always use Joules. More common in thermodynamics is the calorie: By definition 1 calorie is the amount of heat required to change the temperature of 1 gram of water 1°C. ...
energy sources i
... There are three basic mechanisms of heat transfer: Thermal conductivity is the heat transfer due to energy transfer by micro particles Molecules, atoms, electrons and other micro particles contained in substance move with rates proportional to their temperature and transfer the energy from zone with ...
... There are three basic mechanisms of heat transfer: Thermal conductivity is the heat transfer due to energy transfer by micro particles Molecules, atoms, electrons and other micro particles contained in substance move with rates proportional to their temperature and transfer the energy from zone with ...
Discovery Education Science Connection
... During a weather forecast, meteorologists often talk about the temperature-humidity index, commonly called the "heat index." The number is a cross between the air temperature and the relative humidity, pinpointing how the body may feel due to both factors. If the temperature is 90° F and the relativ ...
... During a weather forecast, meteorologists often talk about the temperature-humidity index, commonly called the "heat index." The number is a cross between the air temperature and the relative humidity, pinpointing how the body may feel due to both factors. If the temperature is 90° F and the relativ ...
PowerPoint Presentation - Moving to High
... R-value is a measure of apparent thermal conductivity, and thus describes the rate that heat energy is transferred through a material or assembly, regardless of its original source. Performance of a material is a function of it’s R-value, but is also dependent on the temperature difference on either ...
... R-value is a measure of apparent thermal conductivity, and thus describes the rate that heat energy is transferred through a material or assembly, regardless of its original source. Performance of a material is a function of it’s R-value, but is also dependent on the temperature difference on either ...
Heat Transfer: Conduction, Convection and Latent Heat In addition
... The exchanges of energy by radiation, conduction, convection and latent heat can be quite complex, but in the end they all balance ...
... The exchanges of energy by radiation, conduction, convection and latent heat can be quite complex, but in the end they all balance ...
Using Specific Heat to Determine the Identity of an
... Specific heat is the energy needed to raise one gram of a substance by one degree Celsius. Since this value is unique for every substance, it is possible to use specific heat to determine the identity of an unknown substance. According to the law of conservation of energy, the heat within a system m ...
... Specific heat is the energy needed to raise one gram of a substance by one degree Celsius. Since this value is unique for every substance, it is possible to use specific heat to determine the identity of an unknown substance. According to the law of conservation of energy, the heat within a system m ...
Energy Savings Through Radiant Heat
... thermostats in rooms not be used. Even heat distribution addresses heat loss more effectively. And, you will feel greater comfort at lower temperatures, which means you no longer have to force yourself to turn down the thermostat to save money, you will do it automatically to be comfortable. Heat lo ...
... thermostats in rooms not be used. Even heat distribution addresses heat loss more effectively. And, you will feel greater comfort at lower temperatures, which means you no longer have to force yourself to turn down the thermostat to save money, you will do it automatically to be comfortable. Heat lo ...
Exercise No. 1 - People(dot)tuke(dot)
... by 4 °C. Which one of these materials has greater specific heat capacity? Heat per mass unit, that is added to material, needed for change of its state, is named heat of transformation, eventually latent heat L. Q = L⋅m Most commonly we deal wit the heat of vaporization Lv, what is amount of energy ...
... by 4 °C. Which one of these materials has greater specific heat capacity? Heat per mass unit, that is added to material, needed for change of its state, is named heat of transformation, eventually latent heat L. Q = L⋅m Most commonly we deal wit the heat of vaporization Lv, what is amount of energy ...
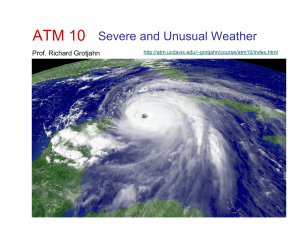
Lecture 2 - Richard Grotjahn
... Ideal Gas Law: Collapsing Jug • The “Gas Law” explains how a jug may collapse when the air inside cools. • T =Temperature, P =Pressure, R=constant ...
... Ideal Gas Law: Collapsing Jug • The “Gas Law” explains how a jug may collapse when the air inside cools. • T =Temperature, P =Pressure, R=constant ...
Chapter 11 Notes
... heat it takes to raise one gram of water by one degree celsius • (note: this is different from a food Calorie, which is actually 1 kilocalorie) • Joules (SI)- 4.184 Joules = 1 calorie ...
... heat it takes to raise one gram of water by one degree celsius • (note: this is different from a food Calorie, which is actually 1 kilocalorie) • Joules (SI)- 4.184 Joules = 1 calorie ...
Page 45a of James Watt`s Laboratory Notebook
... subtract heat given by cone 143 x 72 = 10296 ÷ by 2/3 ...
... subtract heat given by cone 143 x 72 = 10296 ÷ by 2/3 ...
Thermodynamics
... because they can carry away heat quickly and exchange it with the environment. The highest value of k for any natural substance is diamond at 2,200 W/m·K. Other materials are useful because they are extremely poor conductors of heat; this property is referred to as thermal resistance, or R-value, wh ...
... because they can carry away heat quickly and exchange it with the environment. The highest value of k for any natural substance is diamond at 2,200 W/m·K. Other materials are useful because they are extremely poor conductors of heat; this property is referred to as thermal resistance, or R-value, wh ...
Water is able to absorb a high amount of heat before
... The resistance to sudden temperature changes makes water an excellent habitat, allowing organisms to survive without experiencing wide temperature fluctuation. Furthermore, because many organisms are mainly composed of water, the property of high heat capacity allows highly regulated internal body t ...
... The resistance to sudden temperature changes makes water an excellent habitat, allowing organisms to survive without experiencing wide temperature fluctuation. Furthermore, because many organisms are mainly composed of water, the property of high heat capacity allows highly regulated internal body t ...
Specific Heat of Metals Make Up Directions
... 1. Go to the website listed above. Read the information and then scroll down to the applet. Uses JAVA so may need to update or allow JAVA to run. Part 1 is Iron and part 2 is Copper. 2. Use the mass of the water and the metal from your data table below. 3. Using the thermometer on the screen, record ...
... 1. Go to the website listed above. Read the information and then scroll down to the applet. Uses JAVA so may need to update or allow JAVA to run. Part 1 is Iron and part 2 is Copper. 2. Use the mass of the water and the metal from your data table below. 3. Using the thermometer on the screen, record ...
Chapter 7 Thermal and Energy Systems
... • Energy is needed to accelerate an object, stretch it, heat it, and elevate it. • In the internal-combustion engine of Figure, diesel fuel is burned to release thermal energy. • The engine converts thermal energy into the rotation of its crankshaft and ultimately into the motion of a ...
... • Energy is needed to accelerate an object, stretch it, heat it, and elevate it. • In the internal-combustion engine of Figure, diesel fuel is burned to release thermal energy. • The engine converts thermal energy into the rotation of its crankshaft and ultimately into the motion of a ...