Midterm Review 4
... Chemistry: First Semester Exam Prep #4 (Choose the BEST answer.) 52. The most stable atoms are those of the a. metals b. metalloids c. noble gases d. nonmetals 53. The ion with a charge of +1 and the same electron configuration as argon is a. potassium b. sodium c. neon d. magnesium 54. The tendenc ...
... Chemistry: First Semester Exam Prep #4 (Choose the BEST answer.) 52. The most stable atoms are those of the a. metals b. metalloids c. noble gases d. nonmetals 53. The ion with a charge of +1 and the same electron configuration as argon is a. potassium b. sodium c. neon d. magnesium 54. The tendenc ...
Atoms, Ions and Molecules
... All atoms of a given element show the same chemical properties. 2. Atoms of different elements have different properties. In an ordinary chemical reaction, no atom of any element disappears or is changed into an atom of another element. 3. Compounds are formed when atoms of two or more elements comb ...
... All atoms of a given element show the same chemical properties. 2. Atoms of different elements have different properties. In an ordinary chemical reaction, no atom of any element disappears or is changed into an atom of another element. 3. Compounds are formed when atoms of two or more elements comb ...
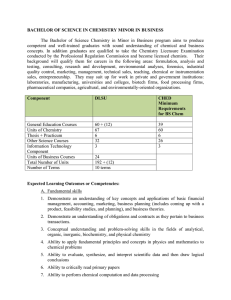
BACHELOR OF SCIENCE IN CHEMISTRY MINOR IN BUSINESS
... This course covers topics in metabolism. The areas to be discussed include bioenergetics, the design and regulation of metabolic pathways and specific molecular processes involved in the synthesis and degradation of major cellular components—the carbohydrates, lipids, proteins and nucleic acid. 3 un ...
... This course covers topics in metabolism. The areas to be discussed include bioenergetics, the design and regulation of metabolic pathways and specific molecular processes involved in the synthesis and degradation of major cellular components—the carbohydrates, lipids, proteins and nucleic acid. 3 un ...
Wolfram, Ch 12
... • Reducing computation work plays an important role in “traditional science” • Even with all information and rules there is irreducible amount of work to do. • “There are many common systems whose behavior cannot in the end be determined at all except by something like an explicit simulation” ??? • ...
... • Reducing computation work plays an important role in “traditional science” • Even with all information and rules there is irreducible amount of work to do. • “There are many common systems whose behavior cannot in the end be determined at all except by something like an explicit simulation” ??? • ...
Chem 115 POGIL Worksheet
... H2 react directly with one molecule of O2 in forming two molecules of H2O. Indeed, the mechanism by which this reaction occurs is less direct. A skeletal equation is a reaction equation that has not yet been balanced. It is just a statement of what the reactants and products are. We will often use a ...
... H2 react directly with one molecule of O2 in forming two molecules of H2O. Indeed, the mechanism by which this reaction occurs is less direct. A skeletal equation is a reaction equation that has not yet been balanced. It is just a statement of what the reactants and products are. We will often use a ...
Russian Doll Renormalization Group and Superconductivity
... values and the cycle repeats itself. Thus if one decreases the size of the system by a specific factor that depends on the coupling constants, one recovers the initial system, much like a Russian doll, or quantum version of the Mandelbrot set. Bedaque, Hammer and Van Kolck observed this behavior in ...
... values and the cycle repeats itself. Thus if one decreases the size of the system by a specific factor that depends on the coupling constants, one recovers the initial system, much like a Russian doll, or quantum version of the Mandelbrot set. Bedaque, Hammer and Van Kolck observed this behavior in ...
CHM 103 Lecture 11 S07
... A. increasing the temperature. B. removing some of the reactants. C. adding a catalyst. D. placing the reaction flask in ice. E. increasing the concentration of one of the ...
... A. increasing the temperature. B. removing some of the reactants. C. adding a catalyst. D. placing the reaction flask in ice. E. increasing the concentration of one of the ...
Numerical Methods in Engineering with Python 3
... Most engineers are not programmers, but problem solvers. They want to know what methods can be applied to a given problem, what their strengths and pitfalls are, and how to implement them. Engineers are not expected to write computer code for basic tasks from scratch; they are more likely to use fun ...
... Most engineers are not programmers, but problem solvers. They want to know what methods can be applied to a given problem, what their strengths and pitfalls are, and how to implement them. Engineers are not expected to write computer code for basic tasks from scratch; they are more likely to use fun ...
homework assignment 2 - the Petersen Home Page
... 4. Cyclist Floyd Landis was recently disqualified as the 2006 champion of the Tour de France because of a higher than normal level of the male hormone testosterone found during subsequent drug tests. Testosterone contains only C, H, and O. If the complete combustion of 25.00 mg of testrosterone prod ...
... 4. Cyclist Floyd Landis was recently disqualified as the 2006 champion of the Tour de France because of a higher than normal level of the male hormone testosterone found during subsequent drug tests. Testosterone contains only C, H, and O. If the complete combustion of 25.00 mg of testrosterone prod ...
02_Lecture_Presentation
... • In a nonpolar covalent bond, the atoms share the electron equally • In a polar covalent bond, one atom is more electronegative, and the atoms do not share the electron equally • Unequal sharing of electrons causes a partial positive or negative charge for each atom or molecule ...
... • In a nonpolar covalent bond, the atoms share the electron equally • In a polar covalent bond, one atom is more electronegative, and the atoms do not share the electron equally • Unequal sharing of electrons causes a partial positive or negative charge for each atom or molecule ...
Convergence of Newton-like methods for solving systems of
... The analysis of Newton-like methods given in this paper is essentially based on the Newton-Kantorovich Theorem (Kantorovich [51) and its extension given by Ortega and Rheinboldt [6]. In our approach the dependence of the a s y m p t o t i c order of convergence on the error in M k as an approximatio ...
... The analysis of Newton-like methods given in this paper is essentially based on the Newton-Kantorovich Theorem (Kantorovich [51) and its extension given by Ortega and Rheinboldt [6]. In our approach the dependence of the a s y m p t o t i c order of convergence on the error in M k as an approximatio ...
Chemical Bonding
... P2, you can assume that third period diatomics form valence molecular orbitals similar to second period diatomics but with n=3) and the following heteronuclear diatomic species, CO, NO and BN (assume the same energy level diagram as Be2 to N2). Determine the bond order of each species. Indicate any ...
... P2, you can assume that third period diatomics form valence molecular orbitals similar to second period diatomics but with n=3) and the following heteronuclear diatomic species, CO, NO and BN (assume the same energy level diagram as Be2 to N2). Determine the bond order of each species. Indicate any ...
Chemistry
... Standard 8. Classifying materials Students will: 8 – 1 Understand the difference between a pure substance, a homogenous mixture and a heterogeneous mixture. Know several methods to separate the components of a mixture and the elements in a compound. 8 – 2 Know the structure of an atom (e.g. negative ...
... Standard 8. Classifying materials Students will: 8 – 1 Understand the difference between a pure substance, a homogenous mixture and a heterogeneous mixture. Know several methods to separate the components of a mixture and the elements in a compound. 8 – 2 Know the structure of an atom (e.g. negative ...
detail
... While it does make sense to say that hdnx = dpx dx since we are dealing with infinitesimals, it will be easier to show how these equations balance if we use hd2 nx = dpx dx, d6 n = d2 nx d2 ny d2 nz , and d6 n = 4πp2 d3 pV /h3 . So, if hd2 nx = dpx dx, then we have hd2 ny = dpy dy and hd2 nz = dpz d ...
... While it does make sense to say that hdnx = dpx dx since we are dealing with infinitesimals, it will be easier to show how these equations balance if we use hd2 nx = dpx dx, d6 n = d2 nx d2 ny d2 nz , and d6 n = 4πp2 d3 pV /h3 . So, if hd2 nx = dpx dx, then we have hd2 ny = dpy dy and hd2 nz = dpz d ...
10 IB Chemistry Assessment Statements 2009 Revised
... Int, aim 8: Today, we may be starting to experience the consequences of using fossil fuels as our main source of energy. There is a vast range of products that can be derived from fossil fuels as a result of carbon’s rich chemistry. This raises the question “are they too valuable to burn?”. ...
... Int, aim 8: Today, we may be starting to experience the consequences of using fossil fuels as our main source of energy. There is a vast range of products that can be derived from fossil fuels as a result of carbon’s rich chemistry. This raises the question “are they too valuable to burn?”. ...
HW / Unit 2
... 6. Fill in the missing parts of the following nuclear equations and identify the type of radiation emitted: a. 21082Pb 21083Bi + ____ b. 146C ____ + 0-1e c. 22688Ra ____ + 42He + gamma ray HW #2.7 1. Explain what each of the following scientists contributed to atomic theory. a. Niels Bohr b. E ...
... 6. Fill in the missing parts of the following nuclear equations and identify the type of radiation emitted: a. 21082Pb 21083Bi + ____ b. 146C ____ + 0-1e c. 22688Ra ____ + 42He + gamma ray HW #2.7 1. Explain what each of the following scientists contributed to atomic theory. a. Niels Bohr b. E ...
1 - New Age International
... Reducing agent is a compound which decreases the oxidation number of an element of the reduced species. It is a species getting oxidised. 25. Balancing of redox equations: Two methods are adopted: (i) ion electron method, (ii) oxidation state method. (i) Ion electron method: The various steps in usi ...
... Reducing agent is a compound which decreases the oxidation number of an element of the reduced species. It is a species getting oxidised. 25. Balancing of redox equations: Two methods are adopted: (i) ion electron method, (ii) oxidation state method. (i) Ion electron method: The various steps in usi ...
dimensional analysis
... Dimensional Analysis • Many problems in Chemistry involve using relationships to convert one unit of measurement to another • Conversion Factors are relationships between two units May be exact or measured Both parts of the conversion factor have the same number of significant figures Tro's Introd ...
... Dimensional Analysis • Many problems in Chemistry involve using relationships to convert one unit of measurement to another • Conversion Factors are relationships between two units May be exact or measured Both parts of the conversion factor have the same number of significant figures Tro's Introd ...
Chemistry Syllabus
... 2e. Compare the properties of compounds according to their type of bonding. (DOK 1) Covalent, ionic, and metallic bonding Polar and non-polar covalent bonding Valence electrons and bonding atoms 2f. Compare different types of intermolecular forces and explain the relationship between intermole ...
... 2e. Compare the properties of compounds according to their type of bonding. (DOK 1) Covalent, ionic, and metallic bonding Polar and non-polar covalent bonding Valence electrons and bonding atoms 2f. Compare different types of intermolecular forces and explain the relationship between intermole ...