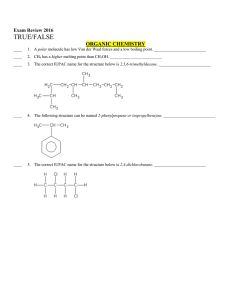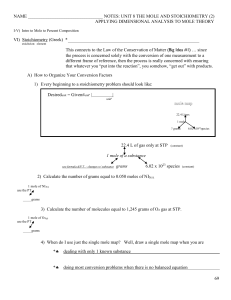
NAME NOTES: UNIT 8 THE MOLE AND STOICHIOMETRY (2
... Much of what you experience deals with "aqueous solutions". Apple juice, milk, soda, lake water, ocean water, tap water are all examples (complex and simple) of aqueous solutions. As in all mixtures, an aqueous solution is a physical combination of a SOLVENT and a SOLUTE. The solvent is the substanc ...
... Much of what you experience deals with "aqueous solutions". Apple juice, milk, soda, lake water, ocean water, tap water are all examples (complex and simple) of aqueous solutions. As in all mixtures, an aqueous solution is a physical combination of a SOLVENT and a SOLUTE. The solvent is the substanc ...
File
... Chemistry 2202 - Unit 1 Test 2 Part 1: For each item, circle the letter corresponding to your choice. ...
... Chemistry 2202 - Unit 1 Test 2 Part 1: For each item, circle the letter corresponding to your choice. ...
29 Sept 08 - Seattle Central
... • Chemistry is the study of the rearrangement of matter due to the flow of energy. • In a chemical reaction, some bonds are broken and others are formed, resulting in a reorganization of the atoms. • Atoms are neither created or destroyed in a chemical reaction! When methane (CH4) reacts with oxygen ...
... • Chemistry is the study of the rearrangement of matter due to the flow of energy. • In a chemical reaction, some bonds are broken and others are formed, resulting in a reorganization of the atoms. • Atoms are neither created or destroyed in a chemical reaction! When methane (CH4) reacts with oxygen ...
Reactions in Aqueous Solution
... products of a given reaction were the only chemical species present. In reality, however, virtually every chemical reaction that takes place within and around us, such as the oxidation of foods to generate energy or the treatment of an upset stomach with an antacid tablet, occur in solution. In fact ...
... products of a given reaction were the only chemical species present. In reality, however, virtually every chemical reaction that takes place within and around us, such as the oxidation of foods to generate energy or the treatment of an upset stomach with an antacid tablet, occur in solution. In fact ...
Chapter 4 "Reactions in Aqueous Solution"
... products of a given reaction were the only chemical species present. In reality, however, virtually every chemical reaction that takes place within and around us, such as the oxidation of foods to generate energy or the treatment of an upset stomach with an antacid tablet, occur in solution. In fact ...
... products of a given reaction were the only chemical species present. In reality, however, virtually every chemical reaction that takes place within and around us, such as the oxidation of foods to generate energy or the treatment of an upset stomach with an antacid tablet, occur in solution. In fact ...
Stoichiometric Calculations
... 16 What is the largest number of of Li3 N formula units that could result from reacting 6 N2 molecules? 6 Li (s) + N2 (g) --# 2 Li3 N (s) ...
... 16 What is the largest number of of Li3 N formula units that could result from reacting 6 N2 molecules? 6 Li (s) + N2 (g) --# 2 Li3 N (s) ...
Chapter 2 1.Certain gases in the 293K and 9.97 × 104Pa when the
... 2s or 2p,is the energy required equal? If it is the helium atom then what will happen? Answer : The extranuclear electron from the ground state of hydrogen atoms excited to the 2s or 2p level, their consumption of energy is equal, because the hydrogen atom is a single-electron atom, 2s and 2p electr ...
... 2s or 2p,is the energy required equal? If it is the helium atom then what will happen? Answer : The extranuclear electron from the ground state of hydrogen atoms excited to the 2s or 2p level, their consumption of energy is equal, because the hydrogen atom is a single-electron atom, 2s and 2p electr ...
MEDICAL CHEMISTRY STUDY GUIDE
... Solution is a homogeneous mixture of two or more substances, consisting of ions or molecules. This term is usually used to describe homogeneous mixtures of two or more liquids or of a liquid and one or more solids. Solutions may exist as gases, liquids, or solids. Nonreactive gases can mix in all pr ...
... Solution is a homogeneous mixture of two or more substances, consisting of ions or molecules. This term is usually used to describe homogeneous mixtures of two or more liquids or of a liquid and one or more solids. Solutions may exist as gases, liquids, or solids. Nonreactive gases can mix in all pr ...
Solutions to Exercises
... 6.31 a. The heat lost by the metal is equal to the heat gained by the water. Since q = s x m x t, the heat gained by the water is directly proportional to t. Since t is larger for metal A, it lost more heat. Now, each metal has the same mass and t, so the specific heat is directly proportional t ...
... 6.31 a. The heat lost by the metal is equal to the heat gained by the water. Since q = s x m x t, the heat gained by the water is directly proportional to t. Since t is larger for metal A, it lost more heat. Now, each metal has the same mass and t, so the specific heat is directly proportional t ...
Chapter 4 Chemical Quantities and Aqueous Reactions
... Electrolytes and Nonelectrolytes • in order to conduct electricity, a material must have charged particles that are able to flow • electrolyte solutions all contain ions dissolved in the water – ionic compounds are electrolytes because they all dissociate into their ions when they dissolve ...
... Electrolytes and Nonelectrolytes • in order to conduct electricity, a material must have charged particles that are able to flow • electrolyte solutions all contain ions dissolved in the water – ionic compounds are electrolytes because they all dissociate into their ions when they dissolve ...
AQA A-level Chemistry
... and products in a chemical reaction. The vertical (y) axis is enthalpy but not ∆H. The horizontal (x) axis is progress of reaction, reaction coordinate or extent of reaction. Two horizontal lines are drawn and labelled with the names or formulae of reactants and products. These represent the enthalp ...
... and products in a chemical reaction. The vertical (y) axis is enthalpy but not ∆H. The horizontal (x) axis is progress of reaction, reaction coordinate or extent of reaction. Two horizontal lines are drawn and labelled with the names or formulae of reactants and products. These represent the enthalp ...
The polydentate ligands include polyaminopolycarbonic acids, such
... Bidentate ligands, e.g. anions of diprotoic acids: SO42-, SO32-, CO32-, C2O42, ethylenediamine molecule NH2-CH2-CH2-NH2 (abbreviated - en) and most of the amino acids react with two complexing agents. The tridentate ligands include aspartic (2-aminobutandioic) acid: ...
... Bidentate ligands, e.g. anions of diprotoic acids: SO42-, SO32-, CO32-, C2O42, ethylenediamine molecule NH2-CH2-CH2-NH2 (abbreviated - en) and most of the amino acids react with two complexing agents. The tridentate ligands include aspartic (2-aminobutandioic) acid: ...
National German competition
... of the first series of measurements. Check for a reaction of zero, first and second order. How can the reaction order be harmonized with the reaction equation? d) Calculate the rate constant for the second series of measurements. What is the activation energy of the hydrolysis? e) What function does ...
... of the first series of measurements. Check for a reaction of zero, first and second order. How can the reaction order be harmonized with the reaction equation? d) Calculate the rate constant for the second series of measurements. What is the activation energy of the hydrolysis? e) What function does ...
Ans:- (i) Gluconic acid - Kendriya Vidyalaya No.2, Kribhco, Surat
... Ans: Negative type of deviation is present. In the negative deviation the solute-solution (A-A) interaction and solvent-solvent (B-B) interaction will be weaker than solute-solvent(A-B ) interaction. Since the new forces are stronger therefore heat is evolved and solution becomes warm. Q13. Which ty ...
... Ans: Negative type of deviation is present. In the negative deviation the solute-solution (A-A) interaction and solvent-solvent (B-B) interaction will be weaker than solute-solvent(A-B ) interaction. Since the new forces are stronger therefore heat is evolved and solution becomes warm. Q13. Which ty ...
coordination compounds
... Bidentate ligands, e.g. anions of diprotoic acids: SO42-, SO32-, CO32-, C2O42, ethylenediamine molecule NH2-CH2-CH2-NH2 (abbreviated - en) and most of the amino acids react with two complexing agents. The tridentate ligands include aspartic (2-aminobutandioic) acid: ...
... Bidentate ligands, e.g. anions of diprotoic acids: SO42-, SO32-, CO32-, C2O42, ethylenediamine molecule NH2-CH2-CH2-NH2 (abbreviated - en) and most of the amino acids react with two complexing agents. The tridentate ligands include aspartic (2-aminobutandioic) acid: ...