Chapter 4: Quantities of Reactants and Products
... Check your answers: A properly balanced equation has the same numbers of atoms of each type (4 C, 12 H, and 14 O) in the products and reactants. The law of conservation of mass says that masses of the reactants must add up to the masses of the products, 284.16 g. This looks right. 12. Define the pro ...
... Check your answers: A properly balanced equation has the same numbers of atoms of each type (4 C, 12 H, and 14 O) in the products and reactants. The law of conservation of mass says that masses of the reactants must add up to the masses of the products, 284.16 g. This looks right. 12. Define the pro ...
Stoichiometry
... Reactions in solution: given the molarity and the volume of the reactants, calculate the amount of product produced or the amount of reactant required to react. Molarity; preparation of solutions. ...
... Reactions in solution: given the molarity and the volume of the reactants, calculate the amount of product produced or the amount of reactant required to react. Molarity; preparation of solutions. ...
Quantitative Chemical Analysis
... One of our most pressing problems is the need for sources of energy to replace oil. The chart at the right shows that world production of oil per capita has probably already peaked. Oil will play a decreasing role as an energy source and should be more valuable as a raw material than as a fuel. Ther ...
... One of our most pressing problems is the need for sources of energy to replace oil. The chart at the right shows that world production of oil per capita has probably already peaked. Oil will play a decreasing role as an energy source and should be more valuable as a raw material than as a fuel. Ther ...
Evaluated kinetic and photochemical data for atmospheric chemistry
... solid particles in Volume V (Crowley et al., 2010). Volume VI of the series extends our work to cover heterogeneous processes involving liquid particles present in the atmosphere. This is done with a view to widening the dissemination and enhancing the accessibility of this evaluated material to the ...
... solid particles in Volume V (Crowley et al., 2010). Volume VI of the series extends our work to cover heterogeneous processes involving liquid particles present in the atmosphere. This is done with a view to widening the dissemination and enhancing the accessibility of this evaluated material to the ...
Chem 12 SM Ch5 Review final new ok revised
... 24. Methane, gasoline, and propane are effective fuel sources because they are all hydrocarbons in which the molecules contain only C–H and C–C bonds. These are high energy bonds that will break and release a large amount of energy upon combustion. 25. We are able to rearrange two chemical equations ...
... 24. Methane, gasoline, and propane are effective fuel sources because they are all hydrocarbons in which the molecules contain only C–H and C–C bonds. These are high energy bonds that will break and release a large amount of energy upon combustion. 25. We are able to rearrange two chemical equations ...
chapter 2 - chemical equations and reaction yields
... CHAPTER 2 - CHEMICAL EQUATIONS AND REACTION YIELDS ...
... CHAPTER 2 - CHEMICAL EQUATIONS AND REACTION YIELDS ...
Problem Set 7
... b. 4.35 x 10-3 moles of zirconium x (91.224g Zr/1mol Zr) =0.397 g Zr c. 3.24 moles of nitrogen gas (N2) oops! Freebie 3.24 mol N2 x (28.0 gN2/1 mol N2) = 90.72 g N2 11) One mole of any gas at STP is 22.4 L. What are the conditions of STP? Why is this previous statement true if CO2 has a different si ...
... b. 4.35 x 10-3 moles of zirconium x (91.224g Zr/1mol Zr) =0.397 g Zr c. 3.24 moles of nitrogen gas (N2) oops! Freebie 3.24 mol N2 x (28.0 gN2/1 mol N2) = 90.72 g N2 11) One mole of any gas at STP is 22.4 L. What are the conditions of STP? Why is this previous statement true if CO2 has a different si ...
Schaum`s Outline of Theory and Problems of
... Make sure you understand the chemical meaning of the terms presented throughout the semester. For example, “significant figures” means something very different in chemical calculations than in economic discussions. Special terms used for the first time in this book will be italicized. Whenever you e ...
... Make sure you understand the chemical meaning of the terms presented throughout the semester. For example, “significant figures” means something very different in chemical calculations than in economic discussions. Special terms used for the first time in this book will be italicized. Whenever you e ...
Stoichiometry
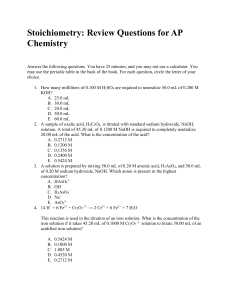
... Calcium reacts with water according to the above reaction. What volume of hydrogen gas, at standard temperature and pressure, is produced from 0.200 mol of calcium? A. 5.60 L B. 2.24 L C. 3.36 L D. 1.12 L E. 4.48 L 18. 2CrO42– + 3SnO22– + H2O → 2 CrO2– + 3 SnO32– + 2 OH– How many moles of OH– form w ...
... Calcium reacts with water according to the above reaction. What volume of hydrogen gas, at standard temperature and pressure, is produced from 0.200 mol of calcium? A. 5.60 L B. 2.24 L C. 3.36 L D. 1.12 L E. 4.48 L 18. 2CrO42– + 3SnO22– + H2O → 2 CrO2– + 3 SnO32– + 2 OH– How many moles of OH– form w ...
Soot Formation Modeling during Hydrocarbon
... In the present work, soot formation was modeled in conditions typical of shock tube experiments. Two different detailed kinetic models (Model-1 and Model-2) were developed. The models were validated by means of a suitable numerical technique (discrete Galerkin method). The gas-phase chemistry of soo ...
... In the present work, soot formation was modeled in conditions typical of shock tube experiments. Two different detailed kinetic models (Model-1 and Model-2) were developed. The models were validated by means of a suitable numerical technique (discrete Galerkin method). The gas-phase chemistry of soo ...
Supplemental Problems
... such as wood, bones, and fossils. While alive, living things take in all the isotopes of carbon, including carbon-14. Carbon-14 undergoes radioactive decay continuously. After an organism dies, the carbon-14 in its body continues to decay. However, its body no longer takes in new carbon-14. Thus, by ...
... such as wood, bones, and fossils. While alive, living things take in all the isotopes of carbon, including carbon-14. Carbon-14 undergoes radioactive decay continuously. After an organism dies, the carbon-14 in its body continues to decay. However, its body no longer takes in new carbon-14. Thus, by ...
Stoichiometric Calculations
... 3 H2 + N2 ® 2 NH3 can be read as: 3 moles of hydrogen gas reacts with 1 mole of nitrogen gas to yield 2 moles of ammonia, or 3 mol H2 + 1 mol N2 to yield 2 mol NH3 The coefficients in the balanced equation give the ratio of moles of reactants and products. Therefore, a balanced chemical equation is ...
... 3 H2 + N2 ® 2 NH3 can be read as: 3 moles of hydrogen gas reacts with 1 mole of nitrogen gas to yield 2 moles of ammonia, or 3 mol H2 + 1 mol N2 to yield 2 mol NH3 The coefficients in the balanced equation give the ratio of moles of reactants and products. Therefore, a balanced chemical equation is ...
4. chemical reactions
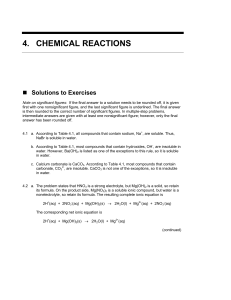
... NaBr is soluble in water. b. According to Table 4.1, most compounds that contain hydroxides, OH-, are insoluble in water. However, Ba(OH)2 is listed as one of the exceptions to this rule, so it is soluble in water. c. Calcium carbonate is CaCO3. According to Table 4.1, most compounds that contain ca ...
... NaBr is soluble in water. b. According to Table 4.1, most compounds that contain hydroxides, OH-, are insoluble in water. However, Ba(OH)2 is listed as one of the exceptions to this rule, so it is soluble in water. c. Calcium carbonate is CaCO3. According to Table 4.1, most compounds that contain ca ...
Stoichiometry
... – Atoms are neither created nor destroyed (they only change bonding partners) – Same atoms are present in the reactants as in the products ...
... – Atoms are neither created nor destroyed (they only change bonding partners) – Same atoms are present in the reactants as in the products ...
College Chemistry
... Dimensional calculations are greatly simplified if a consistent set of units is employed. The three major reference dimensions for mechanics are length, mass, and time, but length can be measured in units of inches, feet, centimeters, meters, etc. Which should be used? The scientific community has m ...
... Dimensional calculations are greatly simplified if a consistent set of units is employed. The three major reference dimensions for mechanics are length, mass, and time, but length can be measured in units of inches, feet, centimeters, meters, etc. Which should be used? The scientific community has m ...