127 - Chimica
... The series of reactions shown in Scheme I were then proven. In this way, all the known dinuclear hydridocarbonyl complexes of rheniumg are chemically related. The structure of the [Re,H,(p-H)(CO),]- anion is shown in Figure 1. It possesses an idealized C2 symmetry and a staggered (torsion angle C13- ...
... The series of reactions shown in Scheme I were then proven. In this way, all the known dinuclear hydridocarbonyl complexes of rheniumg are chemically related. The structure of the [Re,H,(p-H)(CO),]- anion is shown in Figure 1. It possesses an idealized C2 symmetry and a staggered (torsion angle C13- ...
Atomic Model/Theory Webquest - Mr. Smith`s Chemistry Website
... I can describe the crucial contributions of scientists and the critical experiments that led to the development of the modern atomic model. ...
... I can describe the crucial contributions of scientists and the critical experiments that led to the development of the modern atomic model. ...
ppt
... Must include both 2s and 2p orbitals in making our MOs Only orbitals that are close in energy will form MOs We have 2 (2s) orbitals and 6 (2p) orbitals 8 atomic orbitals 2s orbitals overlap in the same way as the 1s orbitals of H2 2p orbitals overlap in same orientations as in Valence bond theory: ...
... Must include both 2s and 2p orbitals in making our MOs Only orbitals that are close in energy will form MOs We have 2 (2s) orbitals and 6 (2p) orbitals 8 atomic orbitals 2s orbitals overlap in the same way as the 1s orbitals of H2 2p orbitals overlap in same orientations as in Valence bond theory: ...
Chapter 6 Electronic Structure of Atoms
... Magnetic Properties of Atoms Although an electron behaves like a tiny magnet, two electrons that are opposite in spin cancel each ...
... Magnetic Properties of Atoms Although an electron behaves like a tiny magnet, two electrons that are opposite in spin cancel each ...
Balancing Chemical Equations
... 3. You can only add coefficients a. coefficients are numbers that go in front of the chemical formula b. 3 FeCl2 + 2 Al the 3 and 2 are coefficients.. ...
... 3. You can only add coefficients a. coefficients are numbers that go in front of the chemical formula b. 3 FeCl2 + 2 Al the 3 and 2 are coefficients.. ...
atomic mass
... • Consists of two types of particles • Proton: Positively charged subatomic particle – Number defines the element • Neutron: Electrically neutral subatomic particle © 2014 W. W. Norton Co., Inc. ...
... • Consists of two types of particles • Proton: Positively charged subatomic particle – Number defines the element • Neutron: Electrically neutral subatomic particle © 2014 W. W. Norton Co., Inc. ...
Byron High School
... the momentum of a particle is known, the less precisely is its position known: ...
... the momentum of a particle is known, the less precisely is its position known: ...
Chapter 5 Powerpoint
... However, not all models are physical. In fact, several theoretical models of the atom have been developed over the last few hundred years. You will learn about the currently accepted model of how electrons behave in atoms. ...
... However, not all models are physical. In fact, several theoretical models of the atom have been developed over the last few hundred years. You will learn about the currently accepted model of how electrons behave in atoms. ...
In order to grasp the concept of molar mass calculations it is
... chemical compounds. Molar masses of common chemical compounds that you might find in the chemistry laboratory can range between 18 grams/mole for compounds like water to hundreds of grams per mole for more complex chemical compounds. The lightest possible chemical that one can have under normal cond ...
... chemical compounds. Molar masses of common chemical compounds that you might find in the chemistry laboratory can range between 18 grams/mole for compounds like water to hundreds of grams per mole for more complex chemical compounds. The lightest possible chemical that one can have under normal cond ...
Document
... This is because graphite has free(delocalised electrons) HT Graphite has free electrons because each carbon atom is covalently bonded to 3 others leaving 1 electron free per carbon atom. Graphite has strong covalent bonds and weak intermolecular forces between layers. Graphite is slippery and can ru ...
... This is because graphite has free(delocalised electrons) HT Graphite has free electrons because each carbon atom is covalently bonded to 3 others leaving 1 electron free per carbon atom. Graphite has strong covalent bonds and weak intermolecular forces between layers. Graphite is slippery and can ru ...
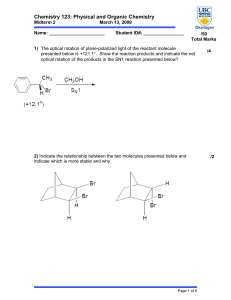
Chemistry 123: Physical and Organic Chemistry
... to -10°C. Describe each step of the process and calculate the amount of energy that would need to flow in or out of the system. At each step indicate if the entropy is increasing or decreasing and under what conditions the reaction would be spontaneous. ...
... to -10°C. Describe each step of the process and calculate the amount of energy that would need to flow in or out of the system. At each step indicate if the entropy is increasing or decreasing and under what conditions the reaction would be spontaneous. ...
naming-and-formulas-chem-1-ab
... the name of each element to indicate the NUMBER of atoms of the element in the molecule. (If the first element’s prefix is mono-, it will be dropped. For example, monocarbon dioxide (CO2) is simply called carbon dioxide.) ...
... the name of each element to indicate the NUMBER of atoms of the element in the molecule. (If the first element’s prefix is mono-, it will be dropped. For example, monocarbon dioxide (CO2) is simply called carbon dioxide.) ...
“atom”?
... • Atomic structure was thought about, but not well known. It took a few more people to really put things together, and build off of each other’s knowledge to come up with what we know today. ...
... • Atomic structure was thought about, but not well known. It took a few more people to really put things together, and build off of each other’s knowledge to come up with what we know today. ...
Atoms and bonds in molecules and chemical
... 1902 (Lewis, 1916), his cubic atomic model in which the vertices are occupied or not by electrons according to the element’s column in the periodic table. In this way, he established a direct link between electrons and the concept of valence which provides a foundation to Abegg’s valence and counter ...
... 1902 (Lewis, 1916), his cubic atomic model in which the vertices are occupied or not by electrons according to the element’s column in the periodic table. In this way, he established a direct link between electrons and the concept of valence which provides a foundation to Abegg’s valence and counter ...
atoms. - Toolbox Pro
... particles called atoms. • All atoms of a given element have identical properties. • Compounds are formed when atoms of different elements combine with one another in small whole numbers. • The relative numbers and kinds of atoms are constant in a given compound. ...
... particles called atoms. • All atoms of a given element have identical properties. • Compounds are formed when atoms of different elements combine with one another in small whole numbers. • The relative numbers and kinds of atoms are constant in a given compound. ...
09 - Northern Highlands
... You have read that atoms, by themselves or combined with other atoms in molecules, are the building blocks of every type of matter. Even though scientists have only recently been able to see atoms directly, they have been able to infer a model for atomic structure that is based on an enormous amount ...
... You have read that atoms, by themselves or combined with other atoms in molecules, are the building blocks of every type of matter. Even though scientists have only recently been able to see atoms directly, they have been able to infer a model for atomic structure that is based on an enormous amount ...
atoms and elements
... An atom is the smallest particle into which an element can be divided and still maintain the properties of that element. All elements are made of atoms. So what’s an element? What makes one element different from another? Let’s find out! Vocabulary: First things first, let’s look at the structure of ...
... An atom is the smallest particle into which an element can be divided and still maintain the properties of that element. All elements are made of atoms. So what’s an element? What makes one element different from another? Let’s find out! Vocabulary: First things first, let’s look at the structure of ...
FREE Sample Here
... 16) One difference between carbon-12 ( 126 C) and carbon-14 ( 146 C) is that carbon-14 has A) two more protons than carbon-12. B) two more electrons than carbon-12. C) two more neutrons than carbon-12. D) A and C only E) B and C only Answer: C Topic: Concept 2.2 Skill: Knowledge/Comprehension 17) 3 ...
... 16) One difference between carbon-12 ( 126 C) and carbon-14 ( 146 C) is that carbon-14 has A) two more protons than carbon-12. B) two more electrons than carbon-12. C) two more neutrons than carbon-12. D) A and C only E) B and C only Answer: C Topic: Concept 2.2 Skill: Knowledge/Comprehension 17) 3 ...
Lab Stuff - WW-P K
... 4. Compounds can be isomers if they have the same molecular formula, but different structural formulas. 5. Hydrocarbons can be evaluated as possible fuel sources by examining their heats of combustion. 6. Energy values can be inserted into a balanced chemical equation. 7. The specific heat of a subs ...
... 4. Compounds can be isomers if they have the same molecular formula, but different structural formulas. 5. Hydrocarbons can be evaluated as possible fuel sources by examining their heats of combustion. 6. Energy values can be inserted into a balanced chemical equation. 7. The specific heat of a subs ...
Atoms and the Periodic Table
... 3. The ELEMENTS in each GROUP have similar PHYSICAL and CHEMICAL properties because they have the SAME number of VALENCE electrons *a. VALENCE ELECTRON(S) are the ELECTRONS found on the OUTER-MOST portion of the electron CLOUD (energy orbital FARTHEST from the NUCLEUS) of an ATOM, which allows the ...
... 3. The ELEMENTS in each GROUP have similar PHYSICAL and CHEMICAL properties because they have the SAME number of VALENCE electrons *a. VALENCE ELECTRON(S) are the ELECTRONS found on the OUTER-MOST portion of the electron CLOUD (energy orbital FARTHEST from the NUCLEUS) of an ATOM, which allows the ...
Ions - amyschaefer24
... that represent the same element but have a net charge. Formed with an atom gains or loses electrons. Why doesn’t an atom form if we gain ...
... that represent the same element but have a net charge. Formed with an atom gains or loses electrons. Why doesn’t an atom form if we gain ...
Final Exam Study Guide Chapters 1-12
... ____ 15. Which of these equations does not describe an inverse proportionality between x and y? a. xy = k c. y = k/x b. x = k/y d. k = x/y ____ 16. Which of the following is not part of Dalton's atomic theory? a. Atoms cannot be divided, created, or destroyed. b. The number of protons in an atom is ...
... ____ 15. Which of these equations does not describe an inverse proportionality between x and y? a. xy = k c. y = k/x b. x = k/y d. k = x/y ____ 16. Which of the following is not part of Dalton's atomic theory? a. Atoms cannot be divided, created, or destroyed. b. The number of protons in an atom is ...
Project 1: Infrared Spectra of Volcanic Plumes
... Schrödinger equation. The first is that if we are interested in the electron density of a molecule (which tells about bonding and potential reactive sites, among other things), then we can consider that the nuclei are fixed. This assumption is called the Born-Oppenheimer approximation, and it works ...
... Schrödinger equation. The first is that if we are interested in the electron density of a molecule (which tells about bonding and potential reactive sites, among other things), then we can consider that the nuclei are fixed. This assumption is called the Born-Oppenheimer approximation, and it works ...
Chapter 3: Atoms: The Building Blocks of Matter
... Differentiate between ionic and covalent using the chemical formulas. Describe the arrangement of ions in a crystal lattice. List and explain the physical properties of ionic compounds. Draw Lewis diagrams for molecular substances. Differentiate between single, double, and triple covalent bonds. Def ...
... Differentiate between ionic and covalent using the chemical formulas. Describe the arrangement of ions in a crystal lattice. List and explain the physical properties of ionic compounds. Draw Lewis diagrams for molecular substances. Differentiate between single, double, and triple covalent bonds. Def ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.