Chemistry STAAR Review File
... Compiled a Periodic Table of 56 elements based on the periodicity of properties such as molar volume when arranged in order of atomic weight. He wrote out the properties of each element on a different card and spent a great deal of time arranging and rearranging them. He was looking for patterns or ...
... Compiled a Periodic Table of 56 elements based on the periodicity of properties such as molar volume when arranged in order of atomic weight. He wrote out the properties of each element on a different card and spent a great deal of time arranging and rearranging them. He was looking for patterns or ...
n and l - Dr.Divan Fard
... The Periodic Table 01 • The periodic table is the most important organizing principle in chemistry. • Chemical and physical properties of elements in the same group are similar. • All chemical and physical properties vary in a periodic manner, hence the name periodic table. ...
... The Periodic Table 01 • The periodic table is the most important organizing principle in chemistry. • Chemical and physical properties of elements in the same group are similar. • All chemical and physical properties vary in a periodic manner, hence the name periodic table. ...
GCSE Chemistry Textbook sample
... Previously you could have learned: › Elements are made of particles called atoms. › Elements are substances containing only one type of atom – ...
... Previously you could have learned: › Elements are made of particles called atoms. › Elements are substances containing only one type of atom – ...
Ch 4 power point short version.pptx
... It was later proved that electrons do have wave properties like diffraction (bending) and interference (overlapping). Heisenberg uncertainty principle: it is impossible to determine simultaneously both the position and velocity of an electron. In 1926, Austrian physicist Erwin Schrödinger developed ...
... It was later proved that electrons do have wave properties like diffraction (bending) and interference (overlapping). Heisenberg uncertainty principle: it is impossible to determine simultaneously both the position and velocity of an electron. In 1926, Austrian physicist Erwin Schrödinger developed ...
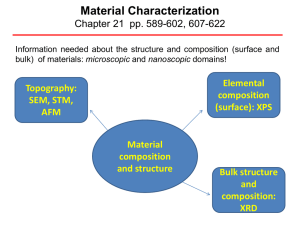
Material Characterization
... X-ray Photoelectron Spectroscopy (XPS) XPS is a surface chemical analysis technique that can be used to analyze the surface chemistry of a material in its "as received" state, or after some treatment XPS detects all elements with an atomic number (Z) of 3 (lithium) and above. It cannot detect hyd ...
... X-ray Photoelectron Spectroscopy (XPS) XPS is a surface chemical analysis technique that can be used to analyze the surface chemistry of a material in its "as received" state, or after some treatment XPS detects all elements with an atomic number (Z) of 3 (lithium) and above. It cannot detect hyd ...
Chapter 5
... can occur when atoms, ions, and molecules collide Activation energy is needed to disrupt electronic configurations Reaction rate is the frequency of collisions with enough energy to bring about a reaction. Reaction rate can be increased by enzymes or by increasing temperature or pressure ...
... can occur when atoms, ions, and molecules collide Activation energy is needed to disrupt electronic configurations Reaction rate is the frequency of collisions with enough energy to bring about a reaction. Reaction rate can be increased by enzymes or by increasing temperature or pressure ...
IB Atomic Structure ppt
... Comparing the I.E. of H and He If we compare the energy required to remove the outermost electron of hydrogen (element number 1) and helium element number 2) we see that approximately twice the energy is needed for helium. As the nuclear charge of helium is twice that of hydrogen we can conclude th ...
... Comparing the I.E. of H and He If we compare the energy required to remove the outermost electron of hydrogen (element number 1) and helium element number 2) we see that approximately twice the energy is needed for helium. As the nuclear charge of helium is twice that of hydrogen we can conclude th ...
Biochemistry Part A PPT
... • Inorganic matter- mostly non living, but essential to living organism • *in general does not contain “C”- Carbon • Exceptions: CO, CO2 • Abundant, and represent raw materials needed to build life ...
... • Inorganic matter- mostly non living, but essential to living organism • *in general does not contain “C”- Carbon • Exceptions: CO, CO2 • Abundant, and represent raw materials needed to build life ...
1.1 - cloudfront.net
... Because atoms are electrically neutral, the number of positively charged protons must be equal to the number of negatively charged electrons. One of Dalton’s points in his atomic theory was that all atoms of a given element are identical in mass. In this section, we will see how this is not strictly ...
... Because atoms are electrically neutral, the number of positively charged protons must be equal to the number of negatively charged electrons. One of Dalton’s points in his atomic theory was that all atoms of a given element are identical in mass. In this section, we will see how this is not strictly ...
LESSON PLAN School : State Senior High School ……………… The
... worked by great scientists, such as Democritus, John Dalton, JJ Thompson, Rutherford, Chadwick, Millikan, Niels Bohr, Schrodinger, de Broglie, and Heisenberg. From those we can determine the atomic structure categorize the element into isotope, isobar, and isoton based of atomic number and the numbe ...
... worked by great scientists, such as Democritus, John Dalton, JJ Thompson, Rutherford, Chadwick, Millikan, Niels Bohr, Schrodinger, de Broglie, and Heisenberg. From those we can determine the atomic structure categorize the element into isotope, isobar, and isoton based of atomic number and the numbe ...
AP CHEMISTRY – Source: 1999 AP Exam CHAPTER 8 PRACTICE
... Ionization Energies for element X (kJ mol-1) First ...
... Ionization Energies for element X (kJ mol-1) First ...
File
... Convert the % composition data to grams. (HINT: If not given grams, use 100 grams of compound to start.) Convert the composition in grams to moles by using molar masses of the elements. (This gives a mole ratio.) Use the numbers from mole ratio to get the smallest possible whole number ...
... Convert the % composition data to grams. (HINT: If not given grams, use 100 grams of compound to start.) Convert the composition in grams to moles by using molar masses of the elements. (This gives a mole ratio.) Use the numbers from mole ratio to get the smallest possible whole number ...
MOLES, MASS, and VOLUME OF A GAS
... Many people feel that they cannot do without their morning cup of coffee. Coffee contains the stimulant caffeine. Analysis shows that it consists of 49.48% Carbon, 5.19% hydrogen, 28.85% nitrogen, and 16.48% Oxygen by mass. Calculate the Empirical formula of Caffeine. ...
... Many people feel that they cannot do without their morning cup of coffee. Coffee contains the stimulant caffeine. Analysis shows that it consists of 49.48% Carbon, 5.19% hydrogen, 28.85% nitrogen, and 16.48% Oxygen by mass. Calculate the Empirical formula of Caffeine. ...
Chemistry Ch3 Honors
... Only a very concentrated (dense) positive charge in a tiny space within the gold atom could possibly repel the fast-moving, positively charged alpha particles enough to reverse the direction of the alpha particles. ...
... Only a very concentrated (dense) positive charge in a tiny space within the gold atom could possibly repel the fast-moving, positively charged alpha particles enough to reverse the direction of the alpha particles. ...
doc: Oxidation Numbers
... that atom would have if the compound was composed of ions. 1. The oxidation number of an atom is zero in a neutral substance that contains atoms of only one element. Thus, the atoms in O2, O3, P4, S8, and aluminum metal all have an oxidation number of 0. 2. The oxidation number of simple ions is equ ...
... that atom would have if the compound was composed of ions. 1. The oxidation number of an atom is zero in a neutral substance that contains atoms of only one element. Thus, the atoms in O2, O3, P4, S8, and aluminum metal all have an oxidation number of 0. 2. The oxidation number of simple ions is equ ...
some basic concepts of chemistry
... different elements are present in a compound in a fixed and definite ratio and this ratio is characteristic of a particular compound. Also, the properties of a compound are different from those of its constituent elements. For example, hydrogen and oxygen are gases whereas the compound formed by the ...
... different elements are present in a compound in a fixed and definite ratio and this ratio is characteristic of a particular compound. Also, the properties of a compound are different from those of its constituent elements. For example, hydrogen and oxygen are gases whereas the compound formed by the ...
What is Chemistry
... •Highly rigid and organized – particles vibrate around fixed points •Nearly incompressible, does not flow ...
... •Highly rigid and organized – particles vibrate around fixed points •Nearly incompressible, does not flow ...
Critical Behavior of Electron Impact Ionization of Atoms
... 3j-symbols. Finally, the electron impact ionization cross section can be evaluated using the calculated scattering amplitude fB : Z ...
... 3j-symbols. Finally, the electron impact ionization cross section can be evaluated using the calculated scattering amplitude fB : Z ...
Chapter 1 The Periodic Table - Beck-Shop
... Mendeleev left a number of gaps in the table. All of the elements were placed in order of increasing atomic weight. III Using his table Mendeleev predicted the existence of some then unknown elements as well as their properties. IV The elements with similar chemical properties were placed in the ...
... Mendeleev left a number of gaps in the table. All of the elements were placed in order of increasing atomic weight. III Using his table Mendeleev predicted the existence of some then unknown elements as well as their properties. IV The elements with similar chemical properties were placed in the ...
Topic 5 Reacting masses and chemical equations notes
... Because of the incredibly small size of atoms, it is not possible to weigh them directly. Instead the masses of atoms are related to an arbitrary standard; for this purpose the mass of one atom of 12C is taken to be 12.0000. The mass of any other atom compared to that of the carbon atom is called it ...
... Because of the incredibly small size of atoms, it is not possible to weigh them directly. Instead the masses of atoms are related to an arbitrary standard; for this purpose the mass of one atom of 12C is taken to be 12.0000. The mass of any other atom compared to that of the carbon atom is called it ...
Chapter 15 - cloudfront.net
... change is equal to the mass of all substances that remain after the change • Suppose logs in a fireplace had a mass of 85 kg • After they burn, all you see is ashes. If you weigh the ...
... change is equal to the mass of all substances that remain after the change • Suppose logs in a fireplace had a mass of 85 kg • After they burn, all you see is ashes. If you weigh the ...
File
... 90. Fluorine is a Group 17 element. Fluorine is the most electronegative and reactive of all elements. It is a pale yellow, corrosive gas, which reacts with practically all organic and inorganic substances. a Draw the Lewis electron-dot structure for an atom of fluorine. b What is the definition (or ...
... 90. Fluorine is a Group 17 element. Fluorine is the most electronegative and reactive of all elements. It is a pale yellow, corrosive gas, which reacts with practically all organic and inorganic substances. a Draw the Lewis electron-dot structure for an atom of fluorine. b What is the definition (or ...
History of molecular theory
In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.The modern concept of molecules can be traced back towards pre-scientific Greek philosophers such as Leucippus who argued that all the universe is composed of atoms and voids. Circa 450 BC Empedocles imagined fundamental elements (fire (20px), earth (20px), air (20px), and water (20px)) and ""forces"" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties. In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies. The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe. A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical substances such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.