Powerpoint - Oregon State University
... N-linked – attached to the R-group amine of asparagine, occurs in Golgi apparatus and ER O-linked – attached to the R-group hydroxides of serine/threonine, occurs in Golgi apparatus ...
... N-linked – attached to the R-group amine of asparagine, occurs in Golgi apparatus and ER O-linked – attached to the R-group hydroxides of serine/threonine, occurs in Golgi apparatus ...
Enzymes
... Create an analogy for an enzyme & substrate on the piece of computer paper. Here’s my example. An enzyme is like a key because it only opens a specific lock and you can use the key over and over again. A substrate is like a lock because it changes forms (reactant Substrate and product). When the ke ...
... Create an analogy for an enzyme & substrate on the piece of computer paper. Here’s my example. An enzyme is like a key because it only opens a specific lock and you can use the key over and over again. A substrate is like a lock because it changes forms (reactant Substrate and product). When the ke ...
Lecture 15a
... •Additions to carboxyl groups Asp, Glu, His, Cys, Tyr, and Lys have pK’s near physiological pH and can assist in general acid-base catalysis. Enzymes arrange several catalytic groups about the substrate to make a concerted catalysis a common mechanism. ...
... •Additions to carboxyl groups Asp, Glu, His, Cys, Tyr, and Lys have pK’s near physiological pH and can assist in general acid-base catalysis. Enzymes arrange several catalytic groups about the substrate to make a concerted catalysis a common mechanism. ...
chapter 18 - rci.rutgers.edu
... the stomach, and then by trypsin, chymotrypsin, and other proteases in the small intestine. Essentially all protein consumed orally is broken down to amino acids, which is why money spent on most "enzyme pills" (like Superoxide Dismutase) is wasted. ...
... the stomach, and then by trypsin, chymotrypsin, and other proteases in the small intestine. Essentially all protein consumed orally is broken down to amino acids, which is why money spent on most "enzyme pills" (like Superoxide Dismutase) is wasted. ...
Characteristics of the caspase-like catalytic domain of
... been proposed in sortases, clan CA proteins from Grampositive bacteria responsible for covalently attaching surface proteins to the cell wall envelope (Zong et al., 2004a,b). In the sortases the canonical His that is part of the catalytic dyad of clan CA proteases has been replaced by an Arg residue ...
... been proposed in sortases, clan CA proteins from Grampositive bacteria responsible for covalently attaching surface proteins to the cell wall envelope (Zong et al., 2004a,b). In the sortases the canonical His that is part of the catalytic dyad of clan CA proteases has been replaced by an Arg residue ...
Exam 1 - Chemistry Courses: About
... chymotrypsin, does not contain aspartate. The pKa values of cysteine and histidine in this active site are 3 and 8 respectively. A. Draw a curve of enzyme activity as a function of pH for this enzyme. What is its approximate ...
... chymotrypsin, does not contain aspartate. The pKa values of cysteine and histidine in this active site are 3 and 8 respectively. A. Draw a curve of enzyme activity as a function of pH for this enzyme. What is its approximate ...
Enzymologie. Jak pracují enzymy
... • typical nucleophilic groups are amino, hydroxyl and thiol groups of AA residues but imidazol group of His or carboxyl group of Asp, Glu can serve as well • electrophilic group of enzymes is usually complex of metal cofactor with substrate • nucleophilic catalysis involves the formation of an inter ...
... • typical nucleophilic groups are amino, hydroxyl and thiol groups of AA residues but imidazol group of His or carboxyl group of Asp, Glu can serve as well • electrophilic group of enzymes is usually complex of metal cofactor with substrate • nucleophilic catalysis involves the formation of an inter ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... (3) Trypsinogen is activated by (a) chymotrypsin (b) trypsin ...
... (3) Trypsinogen is activated by (a) chymotrypsin (b) trypsin ...
Lecture 12
... substrate binding or catalytic activity (e.g., repel negative charges on Glu/Asp or favorable interaction either electrostatic or Hbonding with Arg) • Phosphoryl group capable of 3 H-bonds, highly directional • Phosphorylation is fast enzyme can be turned on/off fast • Example: glycogen phosphoryl ...
... substrate binding or catalytic activity (e.g., repel negative charges on Glu/Asp or favorable interaction either electrostatic or Hbonding with Arg) • Phosphoryl group capable of 3 H-bonds, highly directional • Phosphorylation is fast enzyme can be turned on/off fast • Example: glycogen phosphoryl ...
Chapter 6: An Introduction to Proteins
... histidine residue, with pKa » 6.0, however, can function as a base to abstract the proton from the serine hydroxyl group. The result of transfering the proton from the serine hydroxyl to the histidine imidazole ring is to increase substantially the electronegativity of the serine oxygen, making it a ...
... histidine residue, with pKa » 6.0, however, can function as a base to abstract the proton from the serine hydroxyl group. The result of transfering the proton from the serine hydroxyl to the histidine imidazole ring is to increase substantially the electronegativity of the serine oxygen, making it a ...
enzymes 194 kb enzymes
... D sugar. This displaces the oxygen of the glycosidic bond between D and E, and forms a glycosyl-enzyme intermediate. Glu-35 now donates a proton to the oxygen of C4 of E – this is acid catalysis. To complete the hydrolysis, the now negatively charged Glu-35 activates a water molecule which splits: t ...
... D sugar. This displaces the oxygen of the glycosidic bond between D and E, and forms a glycosyl-enzyme intermediate. Glu-35 now donates a proton to the oxygen of C4 of E – this is acid catalysis. To complete the hydrolysis, the now negatively charged Glu-35 activates a water molecule which splits: t ...
Patrick, An Introduction to Medicinal Chemistry 5e Chapter 3
... histidine and aspartate (or glutamate). Serine would serve as a nucleophile, histidine as an acid/base catalyst and aspartate (or glutamate) as an activating and orientating group. The actual mechanism for the hydrolysis of acetylcholine is described in section 22.12.3.2. ...
... histidine and aspartate (or glutamate). Serine would serve as a nucleophile, histidine as an acid/base catalyst and aspartate (or glutamate) as an activating and orientating group. The actual mechanism for the hydrolysis of acetylcholine is described in section 22.12.3.2. ...
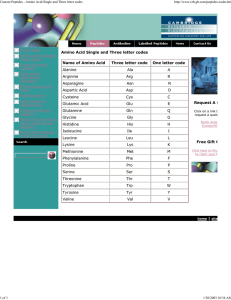
Amino Acid Single and Three letter codes Name of Amino Acid
... Amino Acid Single and Three letter codes Name of Amino Acid ...
... Amino Acid Single and Three letter codes Name of Amino Acid ...
answers_ch04
... 4) A mechanism similar to that described for the hydrolysis of peptide bonds by chymotrypsin (section 4.5.3) would be feasible, involving a catalytic triad of serine, histidine and aspartate. Serine would serve as a nucleophile, histidine as an acid/base catalyst and aspartate as an activating and o ...
... 4) A mechanism similar to that described for the hydrolysis of peptide bonds by chymotrypsin (section 4.5.3) would be feasible, involving a catalytic triad of serine, histidine and aspartate. Serine would serve as a nucleophile, histidine as an acid/base catalyst and aspartate as an activating and o ...
Proteases - Home - KSU Faculty Member websites
... • Protease: is synonymous with peptidase. • Subclass EC 3.4.). 3 = hydrolase 4 = amide bond (peptide bond) • The Peptidases are comprised of two groups of enzymes: – Endopeptidases: cleave peptide bonds at points within the protein. ...
... • Protease: is synonymous with peptidase. • Subclass EC 3.4.). 3 = hydrolase 4 = amide bond (peptide bond) • The Peptidases are comprised of two groups of enzymes: – Endopeptidases: cleave peptide bonds at points within the protein. ...
Document
... B) Oxaloacetate C) a-ketoglutarate D) 3-phosphoglycerate 2. A Roundup Ready plant is one that has been genetically modified so that an enzyme (EPSP synthase) can no longer bind to the active ingredient (glyphosphate) which is a competitive inhibitor of A) shikimate (in the aromatic amino acid pathwa ...
... B) Oxaloacetate C) a-ketoglutarate D) 3-phosphoglycerate 2. A Roundup Ready plant is one that has been genetically modified so that an enzyme (EPSP synthase) can no longer bind to the active ingredient (glyphosphate) which is a competitive inhibitor of A) shikimate (in the aromatic amino acid pathwa ...
Codon Wheel - Your Genome
... Use the codon wheel to translate DNA codons into amino acids. To decode a codon find the first letter of your sequence in the inner circle and work outwards to see the corresponding amino acid. For example: CAT codes for H (Hisitidine). *Please note that this wheel uses the sense DNA codons (5’ to 3 ...
... Use the codon wheel to translate DNA codons into amino acids. To decode a codon find the first letter of your sequence in the inner circle and work outwards to see the corresponding amino acid. For example: CAT codes for H (Hisitidine). *Please note that this wheel uses the sense DNA codons (5’ to 3 ...
Lecture 4 - Sites@UCI
... Forces change in substrate conformation as well! Keep molecules under “strain” to facilitate reaction Active site interactions can stabilize the TS Active site residues can initiate reactions Different enzymes = Different mechanisms ...
... Forces change in substrate conformation as well! Keep molecules under “strain” to facilitate reaction Active site interactions can stabilize the TS Active site residues can initiate reactions Different enzymes = Different mechanisms ...
Enzyme Kinetics
... • can generate a nucleophile by increasing the acidity of a nearby molecule, such as H2O in the hydration of CO2 by carbonic anhydrase. • can bind to substrate, increasing the number of interactions with the enzyme. ...
... • can generate a nucleophile by increasing the acidity of a nearby molecule, such as H2O in the hydration of CO2 by carbonic anhydrase. • can bind to substrate, increasing the number of interactions with the enzyme. ...
Catalytic triad

A catalytic triad refers to the three amino acid residues that function together at the centre of the active site of some hydrolase and transferase enzymes (e.g. proteases, amidases, esterases, acylases, lipases and β-lactamases). An Acid-Base-Nucleophile triad is a common motif for generating a nucleophilic residue for covalent catalysis. The residues form a charge-relay network to polarise and activate the nucleophile, which attacks the substrate, forming a covalent intermediate which is then hydrolysed to regenerate free enzyme. The nucleophile is most commonly a serine or cysteine amino acid, but occasionally threonine. Because enzymes fold into complex three-dimensional structures, the residues of a catalytic triad can be far from each other along the amino-acid sequence (primary structure), however, they are brought close together in the final fold.As well as divergent evolution of function (and even the triad's nucleophile), catalytic triads show some of the best examples of convergent evolution. Chemical constraints on catalysis have led to the same catalytic solution independently evolving in at least 23 separate superfamilies. Their mechanism of action is consequently one of the best studied in biochemistry.