Final Review - Chemistry Courses: About: Department of
... 16. energetics of FA synthesis and degradation 17. nitrogen processing, catabolism of AA 18. medical applications of nucleotide metabolism 19. nucleic acid structure on atomic level ...
... 16. energetics of FA synthesis and degradation 17. nitrogen processing, catabolism of AA 18. medical applications of nucleotide metabolism 19. nucleic acid structure on atomic level ...
Acid-Base Catalysis
... interactions is related to the capacity of surrounding solvent molecules to reduce the attractive forces between chemical groups. Because water is largely excluded from the active site of most enzymes, the local dielectric constant is low. The charge distribution in the relatively anhydrous active s ...
... interactions is related to the capacity of surrounding solvent molecules to reduce the attractive forces between chemical groups. Because water is largely excluded from the active site of most enzymes, the local dielectric constant is low. The charge distribution in the relatively anhydrous active s ...
Enzymes
... glucose even with a low blood [glucose]; a high Km prevents liver from taking up blood glucose when [glucose] is low ...
... glucose even with a low blood [glucose]; a high Km prevents liver from taking up blood glucose when [glucose] is low ...
Exam II
... b. Some permeases can transport their substrates in both directions. c. A symport will transport two molecules in the same direction across a membrane. d. ATP hydrolysis is required. e. An antiport will move substrates in opposite directions across a membrane. ...
... b. Some permeases can transport their substrates in both directions. c. A symport will transport two molecules in the same direction across a membrane. d. ATP hydrolysis is required. e. An antiport will move substrates in opposite directions across a membrane. ...
Model Description Sheet
... Antibiotic-resistant bacteria are common and hard to treat. There is potential to create synthetic antibiotics based on natural products like enduracidin and mannopeptimycin to fight drug resistant bacteria like MRSA. MppP, an enzyme from Streptomyces wadayamensis, is required for the biosynthesis o ...
... Antibiotic-resistant bacteria are common and hard to treat. There is potential to create synthetic antibiotics based on natural products like enduracidin and mannopeptimycin to fight drug resistant bacteria like MRSA. MppP, an enzyme from Streptomyces wadayamensis, is required for the biosynthesis o ...
HERE - Oregon State University
... anion exchange resin. Give the primary structure of the first fragment to elute from the column, if the pH is 6. 2. A scientist working with a protein in dithiothreitol discovers that the protein is very unstable, but when dithiothreitol is absent, the protein is very stable. Explain the most logica ...
... anion exchange resin. Give the primary structure of the first fragment to elute from the column, if the pH is 6. 2. A scientist working with a protein in dithiothreitol discovers that the protein is very unstable, but when dithiothreitol is absent, the protein is very stable. Explain the most logica ...
Enzymes
... ENZYMES ARE SPECIFIC • Every enzyme can only be used for one reaction. Each one can only bond with one substrate • So every time you have a new substrate, you need a new enzyme • This is called being SUBSTRATE SPECIFIC ...
... ENZYMES ARE SPECIFIC • Every enzyme can only be used for one reaction. Each one can only bond with one substrate • So every time you have a new substrate, you need a new enzyme • This is called being SUBSTRATE SPECIFIC ...
Soon you will learn what HIV requires to come to life…
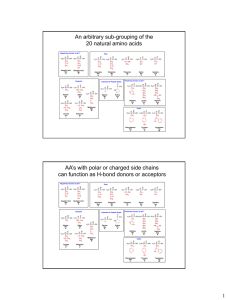
... physiological pH (it can encompass being more than one thing at the same time -- a true intellectual) ...
... physiological pH (it can encompass being more than one thing at the same time -- a true intellectual) ...
In Anfinsen`s experiment, RNAse was denatured with urea and β
... Which of the following is false about the amino acids involved in the active site of chymotrypsin? a) Ser195 acts as a nucleophile. b) His57 acts as a general acid. c) His57 acts as a general base. ...
... Which of the following is false about the amino acids involved in the active site of chymotrypsin? a) Ser195 acts as a nucleophile. b) His57 acts as a general acid. c) His57 acts as a general base. ...
Document
... activated trypsin which may find its way into the ducts, by forming a complex with it Enzyme Y is also exhibiting a protective function, which is activated by traces of active trypsin degrades zymogen The alkaline pH (8.0-9.5) and low Ca2+ concentration in pancreatic secretions promote the degradati ...
... activated trypsin which may find its way into the ducts, by forming a complex with it Enzyme Y is also exhibiting a protective function, which is activated by traces of active trypsin degrades zymogen The alkaline pH (8.0-9.5) and low Ca2+ concentration in pancreatic secretions promote the degradati ...
Chapters 10 and 11 Enzymes Enzymes are specialized proteins that
... higher energy molecule which is closer in energy to the transition state. 3) Covalent Catalysis Attack of a nucleophilic (-) or an electrophilic (+) group in the enzyme active site upon substrate creates a covalent bond between E and S. 4) Proximity Enzymes bring 2 reactants together with the correc ...
... higher energy molecule which is closer in energy to the transition state. 3) Covalent Catalysis Attack of a nucleophilic (-) or an electrophilic (+) group in the enzyme active site upon substrate creates a covalent bond between E and S. 4) Proximity Enzymes bring 2 reactants together with the correc ...
BB 450/550 Exam 1 - Oregon State University
... 2. Name and describe the structure of proteins that gives rise to alpha-helices, folding, and subunit interactions. 3. What are the primary forces stabilizing alpha helices? 4. Using a diagram, clearly illustrate the arrangement of hydrophobic and hydrophilic amino acids in a membrane protein like p ...
... 2. Name and describe the structure of proteins that gives rise to alpha-helices, folding, and subunit interactions. 3. What are the primary forces stabilizing alpha helices? 4. Using a diagram, clearly illustrate the arrangement of hydrophobic and hydrophilic amino acids in a membrane protein like p ...
Ribozyme Catalysis
... • A neutral hydroxyl (e.g. Ser-OH) isn’t a very good nucleophile, but deprotonation would require a strong base • “Catalytic triad”: a glutamate (or aspartate) carboxylate hydrogen-bonds with a histidine’s imidazole group, which increases its basicity enough to assist with removal of serine’s hydro ...
... • A neutral hydroxyl (e.g. Ser-OH) isn’t a very good nucleophile, but deprotonation would require a strong base • “Catalytic triad”: a glutamate (or aspartate) carboxylate hydrogen-bonds with a histidine’s imidazole group, which increases its basicity enough to assist with removal of serine’s hydro ...
File
... digestive system to cope with it. Proteases are used to produce baby food from cow’s milk. The proteases break down milk proteins into amino acids, diminishing the risk of babies developing milk allergies. Particular proteases are also used for the production of hypoallergenic food . These proteases ...
... digestive system to cope with it. Proteases are used to produce baby food from cow’s milk. The proteases break down milk proteins into amino acids, diminishing the risk of babies developing milk allergies. Particular proteases are also used for the production of hypoallergenic food . These proteases ...
Catalytic Strategies
... • Amino acids side chains offer variety of nucleophilic centers for catalysis • These groups readily attack electrophilic centers of substrates, forming covalent enzyme-substrate complexes • The covalent intermediate can be attacked in a second step by water or by a second substrate, forming the des ...
... • Amino acids side chains offer variety of nucleophilic centers for catalysis • These groups readily attack electrophilic centers of substrates, forming covalent enzyme-substrate complexes • The covalent intermediate can be attacked in a second step by water or by a second substrate, forming the des ...
HIV-1 Protease - Illinois State University
... red, while Asp-29, Asp 125, and Asp 129 are green. Also shown are three water molecules, HOH-301, HOH566, HOH608, which play a crucial role in bonding. ...
... red, while Asp-29, Asp 125, and Asp 129 are green. Also shown are three water molecules, HOH-301, HOH566, HOH608, which play a crucial role in bonding. ...
Catalytic triad

A catalytic triad refers to the three amino acid residues that function together at the centre of the active site of some hydrolase and transferase enzymes (e.g. proteases, amidases, esterases, acylases, lipases and β-lactamases). An Acid-Base-Nucleophile triad is a common motif for generating a nucleophilic residue for covalent catalysis. The residues form a charge-relay network to polarise and activate the nucleophile, which attacks the substrate, forming a covalent intermediate which is then hydrolysed to regenerate free enzyme. The nucleophile is most commonly a serine or cysteine amino acid, but occasionally threonine. Because enzymes fold into complex three-dimensional structures, the residues of a catalytic triad can be far from each other along the amino-acid sequence (primary structure), however, they are brought close together in the final fold.As well as divergent evolution of function (and even the triad's nucleophile), catalytic triads show some of the best examples of convergent evolution. Chemical constraints on catalysis have led to the same catalytic solution independently evolving in at least 23 separate superfamilies. Their mechanism of action is consequently one of the best studied in biochemistry.