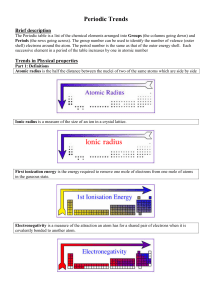
CHEM 1305 - HCC Learning Web
... PART I – Multiple Choice: (3 points each) -------1. What is the term for the value which indicates the number of protons for an atom of a given element? A) Atomic notation? B) Atomic number? C) Atomic mass? D) Mass number? -------2. What is the term for the shorthand description of the arrangement o ...
... PART I – Multiple Choice: (3 points each) -------1. What is the term for the value which indicates the number of protons for an atom of a given element? A) Atomic notation? B) Atomic number? C) Atomic mass? D) Mass number? -------2. What is the term for the shorthand description of the arrangement o ...
Electrons in a Shell - University of California, Berkeley
... In this brief note, we consider the spatial distribution of N>>1 non-relativistic electrons placed inside an empty spherical shell of radius a at zero temperature. This problem was offered as an exercise on the Thomas-Fermi (T-F) model (see, e.g., [1]) in an upper division class in atomic physics (P ...
... In this brief note, we consider the spatial distribution of N>>1 non-relativistic electrons placed inside an empty spherical shell of radius a at zero temperature. This problem was offered as an exercise on the Thomas-Fermi (T-F) model (see, e.g., [1]) in an upper division class in atomic physics (P ...
Spectroscopy - Birmingham City Schools
... depends on orbit jump determines color o Spectra give info about allowed energy levels ...
... depends on orbit jump determines color o Spectra give info about allowed energy levels ...
m ι
... Schrödinger (wave) equation: • An acceptable solution to Schrödinger equation that states the location of an electron at a given point in space and each wave function is associated with a particular energy E. • A plot of distance versus 2 represents an orbital, a probability distribution map of a ...
... Schrödinger (wave) equation: • An acceptable solution to Schrödinger equation that states the location of an electron at a given point in space and each wave function is associated with a particular energy E. • A plot of distance versus 2 represents an orbital, a probability distribution map of a ...
Unit 2 – Electrons and Periodic Behavior Cartoon courtesy of
... • States that orbital of equal energy are each occupied by 1 electron before any orbital is occupied by a 2nd electron. Fill in all the up arrows ...
... • States that orbital of equal energy are each occupied by 1 electron before any orbital is occupied by a 2nd electron. Fill in all the up arrows ...
QUIZ
... d. their outermost energy level is filled 8. All the noble gas elements are _____ a. stable and do not normally react with other elements b. on the left side of the periodic table c. insufferable at parties d. none of the above 9. Chemical bonds are the forces that _______ a. keep electrons in their ...
... d. their outermost energy level is filled 8. All the noble gas elements are _____ a. stable and do not normally react with other elements b. on the left side of the periodic table c. insufferable at parties d. none of the above 9. Chemical bonds are the forces that _______ a. keep electrons in their ...
Word - chemmybear.com
... Atoms tend to lose, gain, or ___________ electrons to complete their valence shells. When a chlorine atom gains an electron, it fills its valence shell forming a negative chloride________. Whenever ionic solids are formed, __________ is involved. An ionic material is composed of positive ions bonded ...
... Atoms tend to lose, gain, or ___________ electrons to complete their valence shells. When a chlorine atom gains an electron, it fills its valence shell forming a negative chloride________. Whenever ionic solids are formed, __________ is involved. An ionic material is composed of positive ions bonded ...
atomic structure sm
... The electrons of a many-electron atom occupy the orbitals in accordance with a series of rules. The Pauli Exclusion Principle: Only two electrons with opposite spin can occupy an atomic orbital. Stated another way, no two electrons have the same 4 quantum numbers n, l, ml, ms. In applying this rule, ...
... The electrons of a many-electron atom occupy the orbitals in accordance with a series of rules. The Pauli Exclusion Principle: Only two electrons with opposite spin can occupy an atomic orbital. Stated another way, no two electrons have the same 4 quantum numbers n, l, ml, ms. In applying this rule, ...
Physics 200 Class #1 Outline
... Light rays reflecting off the different faces of the crystal can interfere with each other. This is similar to thin film interference we spoke about. In the diagram below, we're looking at the path length difference between rays bouncing off the top layer of a crystal and the second layer. The impor ...
... Light rays reflecting off the different faces of the crystal can interfere with each other. This is similar to thin film interference we spoke about. In the diagram below, we're looking at the path length difference between rays bouncing off the top layer of a crystal and the second layer. The impor ...
Knowing the subshells of an electron shell
... Knowing the subshells of an electron shell Fill in the information missing from this table: Some electron shells shell subshells ...
... Knowing the subshells of an electron shell Fill in the information missing from this table: Some electron shells shell subshells ...
QUANTUM NUMBERS
... this was explained by Sommerfeld & Debye (1916) who thought that orbits may exist at varying angles and that the energies may be different when near strong magnets for each value of l, ml can vary from –l to +l (each value represents a different orientation) i.e. if l=1 then ml can be –1,0 or ...
... this was explained by Sommerfeld & Debye (1916) who thought that orbits may exist at varying angles and that the energies may be different when near strong magnets for each value of l, ml can vary from –l to +l (each value represents a different orientation) i.e. if l=1 then ml can be –1,0 or ...
3 Nov 08 - Seattle Central College
... • Naming the electron orbitals is done as follows – n is simply referred to by the quantum number – (0…n - 1) is given a letter value as follows: •0 = s •1 = p •2 = d •3 = f ...
... • Naming the electron orbitals is done as follows – n is simply referred to by the quantum number – (0…n - 1) is given a letter value as follows: •0 = s •1 = p •2 = d •3 = f ...
quantum number
... 1) Pauli principle - No two electrons can have the same set of four quantum numbers. 2) Aufbau principle - Electrons add to the lowest energy available orbital until that orbital is filled. 3) Hund’s rule - Electrons add in such a way as to make as many of the electrons as possible “spin up” (ms = 1 ...
... 1) Pauli principle - No two electrons can have the same set of four quantum numbers. 2) Aufbau principle - Electrons add to the lowest energy available orbital until that orbital is filled. 3) Hund’s rule - Electrons add in such a way as to make as many of the electrons as possible “spin up” (ms = 1 ...
Slide 1
... • For an orbital of principal quantum number n, the value of l can have an integer value from 0 to n – 1. • This gives the subshell notation: ...
... • For an orbital of principal quantum number n, the value of l can have an integer value from 0 to n – 1. • This gives the subshell notation: ...
Electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s2 2s2 2p6.Electronic configurations describe electrons as each moving independently in an orbital, in an average field created by all other orbitals. Mathematically, configurations are described by Slater determinants or configuration state functions.According to the laws of quantum mechanics, for systems with only one electron, an energy is associated with each electron configuration and, upon certain conditions, electrons are able to move from one configuration to another by the emission or absorption of a quantum of energy, in the form of a photon.Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements. The concept is also useful for describing the chemical bonds that hold atoms together. In bulk materials, this same idea helps explain the peculiar properties of lasers and semiconductors.