Exam on Matter through Bonding
... Base your answers to questions 24 through 26 on the information below. Given the heating curve where substance X starts as a solid below its melting point and heat is added uniformly. ...
... Base your answers to questions 24 through 26 on the information below. Given the heating curve where substance X starts as a solid below its melting point and heat is added uniformly. ...
CH7 handout is here.
... 8. Heisenberg uncertainty principle states that we cannot know exactly the position and velocity of an electron both at the same instant. Explain what we studied under ‘position’ and under ‘velocity’. What were the assumptions when studying ‘position’? “velocity”? ...
... 8. Heisenberg uncertainty principle states that we cannot know exactly the position and velocity of an electron both at the same instant. Explain what we studied under ‘position’ and under ‘velocity’. What were the assumptions when studying ‘position’? “velocity”? ...
The Standard Model (SM) describes the fundamental particles of the
... Electromagnetic (EM) – This interaction deals with electrically charged particles. Any particle with electric charge creates an electric field, and if the charge is moving it creates a magnetic field. This interaction is mediated by the exchange of the photon, a boson having no mass or charge. An el ...
... Electromagnetic (EM) – This interaction deals with electrically charged particles. Any particle with electric charge creates an electric field, and if the charge is moving it creates a magnetic field. This interaction is mediated by the exchange of the photon, a boson having no mass or charge. An el ...
Theory of electrons and positrons P A. M. D
... From general philosophical grounds one would at first sight like to have as few kinds of elementary particles as possible, say only one kind, or at most two, and to have all matter built up of these elementary kinds. It appears from the experimental results, though, that there must be more than this ...
... From general philosophical grounds one would at first sight like to have as few kinds of elementary particles as possible, say only one kind, or at most two, and to have all matter built up of these elementary kinds. It appears from the experimental results, though, that there must be more than this ...
3.3 Review Name________________________________ Period_______Date_____________________
... ______ 13. Used Planck’s idea of quantization to explain the line spectrum of hydrogen. ______ 14. Stated that the position and momentum of a moving object cannot be simultaneously measured and known exactly. ______ 15. Labeled each energy level in his atomic model with the principal quantum number, ...
... ______ 13. Used Planck’s idea of quantization to explain the line spectrum of hydrogen. ______ 14. Stated that the position and momentum of a moving object cannot be simultaneously measured and known exactly. ______ 15. Labeled each energy level in his atomic model with the principal quantum number, ...
Chapter 7 Student Learning Map
... duality explanation used to explain light and electrons? What is the relationship between the speed, frequency, and wavelength of electromagnetic radiation? What is the significance of the photoelectric effect in describing the behavior of the electron and light? ...
... duality explanation used to explain light and electrons? What is the relationship between the speed, frequency, and wavelength of electromagnetic radiation? What is the significance of the photoelectric effect in describing the behavior of the electron and light? ...
Particle Physics
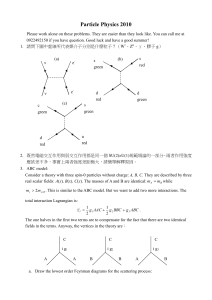
... needed. You can drop all the multiplicative constants. Comment: This is the result for a structure-less scattering. Compare it to the answers in 3c, 3d where there is a propagating mediating particle. From the experimental data, we can tell which the case is. b. Consider the process A( p1 ) B( p2 ...
... needed. You can drop all the multiplicative constants. Comment: This is the result for a structure-less scattering. Compare it to the answers in 3c, 3d where there is a propagating mediating particle. From the experimental data, we can tell which the case is. b. Consider the process A( p1 ) B( p2 ...
Problem set 4
... 900 Watts in a collimated beam in the x̂ direction. What is the force on the source? h2i 2. How many photons from a 100 MHz beam of FM radio waves must an electron absorb before it has gained an energy of 10 eV? h1i 3. Is the discreteness of the energy in an electromagnetic wave more easily detected ...
... 900 Watts in a collimated beam in the x̂ direction. What is the force on the source? h2i 2. How many photons from a 100 MHz beam of FM radio waves must an electron absorb before it has gained an energy of 10 eV? h1i 3. Is the discreteness of the energy in an electromagnetic wave more easily detected ...
Moderne Methoden der Materialcharakterisierung
... Cathode material determines emission current density ...
... Cathode material determines emission current density ...
Tutorial 1 - NUS Physics Department
... particle B, producing C1 , C2 ,) , there is another inertial frame [besides the lab (B at rest) and the CM (PTOT = 0 )] which is sometimes useful. It is called the Breit, or “brick wall,” frame, and it is the system in which A recoils with its momentum reversed (Pafter = - P before ), as though it ...
... particle B, producing C1 , C2 ,) , there is another inertial frame [besides the lab (B at rest) and the CM (PTOT = 0 )] which is sometimes useful. It is called the Breit, or “brick wall,” frame, and it is the system in which A recoils with its momentum reversed (Pafter = - P before ), as though it ...
Alessandro Bettini Introduction to Elementary Particle Physics
... superconducting magnets Bmax~ 9.0 T FNAL and LHC and the HERA proton ring used superconducting magnets There are RF (radio frequency cavities) to maintain the energy. The beam circulates in a vacuum tube and is grouped into “bunches”. Question: Why not continuous beams ? ...
... superconducting magnets Bmax~ 9.0 T FNAL and LHC and the HERA proton ring used superconducting magnets There are RF (radio frequency cavities) to maintain the energy. The beam circulates in a vacuum tube and is grouped into “bunches”. Question: Why not continuous beams ? ...
Quiz 1 Key
... There should be 4 equal lobes in the x y plane. The lobes should not be on the x y axis, but at a point in the middle. ...
... There should be 4 equal lobes in the x y plane. The lobes should not be on the x y axis, but at a point in the middle. ...
Practice Exam III
... 1. Which of the following is a statement of Hess's law? A) If a reaction is carried out in a series of steps, the ΔH for the reaction will equal the sum of the enthalpy changes for the individual steps. B) If a reaction is carried out in a series of steps, the ΔH for the reaction will equal the prod ...
... 1. Which of the following is a statement of Hess's law? A) If a reaction is carried out in a series of steps, the ΔH for the reaction will equal the sum of the enthalpy changes for the individual steps. B) If a reaction is carried out in a series of steps, the ΔH for the reaction will equal the prod ...
Electron scattering

Electron scattering occurs when electrons are deviated from their original trajectory. This is due to the electrostatic forces within matter interaction or, if an external magnetic field is present, the electron may be deflected by the Lorentz force. This scattering typically happens with solids such as metals, semiconductors and insulators; and is a limiting factor in integrated circuits and transistors.The application of electron scattering is such that it can be used as a high resolution microscope for hadronic systems, that allows the measurement of the distribution of charges for nucleons and nuclear structure. The scattering of electrons has allowed us to understand that protons and neutrons are made up of the smaller elementary subatomic particles called quarks.Electrons may be scattered through a solid in several ways:Not at all: no electron scattering occurs at all and the beam passes straight through.Single scattering: when an electron is scattered just once.Plural scattering: when electron(s) scatter several times.Multiple scattering: when electron(s) scatter very many times over.The likelihood of an electron scattering and the proliferance of the scattering is a probability function of the specimen thickness to the mean free path.