* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Slide 1
Atherosclerosis wikipedia , lookup
Rheumatic fever wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Kawasaki disease wikipedia , lookup
Management of multiple sclerosis wikipedia , lookup
Autoimmune encephalitis wikipedia , lookup
Behçet's disease wikipedia , lookup
Anti-nuclear antibody wikipedia , lookup
Pathophysiology of multiple sclerosis wikipedia , lookup
Inflammatory bowel disease wikipedia , lookup
Ankylosing spondylitis wikipedia , lookup
Monoclonal antibody wikipedia , lookup
Rheumatoid arthritis wikipedia , lookup
Globalization and disease wikipedia , lookup
Germ theory of disease wikipedia , lookup
African trypanosomiasis wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
Sjögren syndrome wikipedia , lookup
Neuromyelitis optica wikipedia , lookup
Coeliac disease wikipedia , lookup
IgA nephropathy wikipedia , lookup
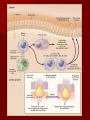
Tissue Transglutaminase, Endomysial Antibodies, and Celiac Disease David Lucas, M.D. CP Conference, January 18, 2008 Celiac Disease Celiac disease is characterized by small intestinal damage with loss of absorptive villi and hyperplasia of crypts typically leading to malabsorption Associated with nutrient deficiencies and increased risk of malignancy (especially intestinal T-cell lymphoma) with chronic disease Precipitated by ingestion of the protein gliadin (component of wheat gluten) and resolves with withdrawal of protein Gliadin initiates mucosal damage via an immunological process in individuals with a genetic predisposition Celiac Disease Mechanism responsible for damage is still under debate Small intestinal biopsy is “gold standard” and reveals flattened/blunted villi IgA antibodies against gliadin and endomysium (a smooth muscle connective tissue) have been the sole serological test used for both diagnosis and therapy control IgA antibodies to endomysium are very specific indicators of celiac disease, suggesting one or more target autoantigens Tissue Transglutaminase Identification In 1997, tissue transglutaminase (tTG) as the unknown endomysial autoantigen was identified tTG – otherwise known as erythrocyte, cellular, endothelial, cytoplasmic, Type II, or liver TG Family of calcium-dependent enzymes that catalyze the crosslinking of proteins resulting in the formation of epsilon-lysine bonds Normally localized within the cytoplasm and released during wound healing, where it interacts with cell surfaces or certain extracellular matrix molecules Tissue Transglutaminase Identification tTG can also crosslink with fibronectin, osteonectin, collagen II, V, and XI, procollagen III, and nidogen Thought to stabilize the provisional extracellular matrix in granulation tissue Also enhanced during apoptosis leading to irreversible crosslinking of intercellular proteins Gliadin proved to be an excellent substrate for tTG Tissue Transglutaminase Identification Indirect immunofluorescence performed with high titer Celiac disease serum placed on monkey esophagus with or without tTG pretreatment Untreated serum revealed a characteristic endomysial immunofluorescence pattern consistent with disease whereas the treated serum pattern nearly completely abolished endomysial immunofluorescence Tissue Transglutaminase Identification This demonstrated that tTG was the predominant, if not sole, endomysial autoantigen An ELISA for the detection of IgA anti-tTG was established Pathogenesis Fundamental disorder is sensitivity to gluten, which is the alcohol-soluble, water-insoluble protein component (gliadin) of wheat and closely related grains (oat, barley, and rye) T-cell mediated chronic inflammatory reaction with autoimmune component Most likely develops as a consequence of a loss of tolerance to gluten Interplay between genetic predisposing factors, the host immune response, and environmental factors is central to disease pathogenesis Pathogenesis When exposed to gluten, the small intestine accumulates intraepithelial CD8+ T cells and large numbers of lamina propria CD4+ T cells, which are sensitized to gliadin Also major histocompatibility complex class II HLA-DQ2 or HLA-DQ8 haplotype Recognition of these peptides by CD4+ T cells leads to secretion of interferon γ, which damages the intestinal wall Epithelial cells secrete large amounts of IL-15 that activates CD8+ T cells and increases the risk of lymphoma development. Thoughts regarding EMA and tTG EMA is nearly 100% specific and is the gold standard in serologic testing for celiac disease The ease of the assay for anti-tTG (ELISA) as opposed to EMA (immunofluorescence) has influenced many reference labs In celiac disease, there are EMA and anti-tTG negative patients, as well as lack of concordance between anti-tTG and EMA in up to one-third of patients A celiac panel for celiac disease should include both EMA and anti-tTG serology Thoughts regarding EMA and tTG IgA EMA using human umbilical cord had a sensitivity of 90% and a specificity of 99% ELISA anti-tTG antibodies using guinea pig liver tTG has shown a sensitivity of 93% and specificity of 95% The introduction of human recombinant tTG has shown to enhance the sensitivity of the anti-tTG test A combination of antibodies should be used – to include anti-tTG and EMA antibodies, with at least one being IgG based (IgA deficient patients) Additional Points 2.6% of patients with celiac disease have selective IgA deficiency – Should have both IgA and IgG base serological screening tests and total IgA if the above is suspected Two further subgroups: – Patients with only IgG-type antibodies to both tTG and EMA, but with normal IgA – Patients with anti-tTG and anti-R1 reticulin antibodies, but lacking typical EMA References Dieterich W, Ehnis T, Bauer M, Donner P, Volta U, Riecken EO, Schuppan D. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med 1997;3:797–801 Dickey W, Hughes DF, McMillan SA. Reliance on serum endomysial antibody testing underestimates the true prevalence of coeliac disease by one fifth. Scand J Gastroenterol 2000;35:181–183 Stern M. Comparative evaluation of serologic tests for celiac disease: a European initiative toward standardization. J Pediatr Gastroenterol Nutr 2000;31:513–519