* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Diffusion & Osmosis
Survey
Document related concepts
Transcript
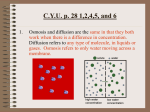
Diffusion & Osmosis Ch 8 Diffusion Definition: Net movement of particles from an area of _______ high concentration to an area of ______ low concentration The smell of popcorn traveling Example: _____________________________ across the room Diffusion results from the _________ random movements of particles (Brownian motion) Diffusion is a ________ SLOW process 3 factors affect the ________ (how rate quickly or slowly) of diffusion Temperature Temp. ________________ If you increase ________ it will move more then ______________________ Conc. Concentration If you increase ________ ________________ then __________________________ it will diffuse more rapidly Pressure Pressure ________________ If you increase ________ then _______________________ it will increase diffusion Eventually… the particles will become evenly distributed _______ The condition in which there is continuous movement, but no overall concentration change is called dynamic equilibrium __________________ The difference in concentration of a substance across a space is called _______________________ concentration gradient High concentration Low concentration In the case with diffusion, particles high are moving from an area of _____ low concentration to an area of _____ concentration; therefore, the substance is said to move ______ with the concentration gradient. Another example of diffusion: Oxygen in the air sacs of lungs diffuses into capillaries Particles constantly move & bounce off each other and other surfaces Osmosis Definition: Diffusion of water across a Semi-permeable membrane _________________ diffusion Osmosis is a special example of __________ Osmosis Example: Absorption of water by plant roots Osmosis It is important to notice that large impermeable molecules do not __________________ H 20 cross the membrane, but _______ molecules do cross during osmosis Osmosis occurs in both plant and animal cells and there are three conditions that typically appear. Isotonic Solution Hypotonic solution Concentration of dissolved substances in the same as the concentration of solution is the _______ dissolved substances inside the cell. Concentration of dissolved substances is less in the solution outside the cell than the _______ concentration inside the cell Hypertonic solution Concentration of dissolved substances is greater outside than the concentration inside ________ the cell ISOTONIC SOLUTION 10% salt HYPOTONIC SOLUTION cell 10% salt 2% salt cell HYPERTONIC SOLUTION 10% salt 25% salt cell 10% salt Normal Cells in Isotonic Solution Cells in Hypertonic Solution Cells in Hypotonic Solution ISOTONIC SOLUTION 10% salt HYPOTONIC SOLUTION cell 10% salt 2% salt cell Equal movement in and out HYPERTONIC SOLUTION 10% salt 25% salt cell More H2O in than out 10% salt More H2O out than in Here is what happens when more water exits or more water enters