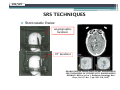
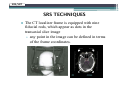
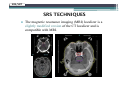
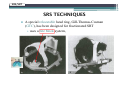
* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Special procedures and techniques in radiotherapy
Industrial radiography wikipedia , lookup
Brachytherapy wikipedia , lookup
Radiation therapy wikipedia , lookup
Nuclear medicine wikipedia , lookup
Backscatter X-ray wikipedia , lookup
Proton therapy wikipedia , lookup
Neutron capture therapy of cancer wikipedia , lookup
Radiation burn wikipedia , lookup
Special procedures and techniques in radiotherapy 講者:蕭安成 物理師 References: 1. The physics of radiation therapy / Faiz M. Khan. - 4th ed. 2. AAPM REPORT NO. 23 TOTAL SKIN ELECTRON THERAPY: TECHNIQUE AND DOSIMETRY 3. AAPM REPORT NO. 17 THE PHYSICAL ASPECTS OF TOTAL AND HALF BODY PHOTON IRRADIATION 4. Stereotactic body radiation therapy: The report of AAPM Task Group 101. Med. Phys. 37 (8), August 2010, 4078-4101 5. AAPM REPORT NO. 54 STEREOTACTIC RADIOSURGERY 2 Special procedures and techniques in radiotherapy Total Body Irradiation Total Skin Irradiation Stereotactic Radiosurgery/Radiotherapy Stereotactic Body Radiation Therapy Total Body Irradiation (TBI) Clinical indication – Total body irradiation (TBI) with megavoltage photon beams is most commonly used as part of the conditioning regimen for bone marrow transplantation, which is used in the treatment of diseases such as leukemia, aplastic anemia, lymphoma, multiple myeloma, autoimmune disease, inborn errors of metabolism, and so on. Total Body Irradiation (TBI) Clinical indication – The role of TBI is to destroy the recipient’s bone marrow and tumor cells, and to immunosuppress the patient sufficiently to avoid rejection of the donor bone marrow transplant – Usually the patient undergoes a chemotherapy conditioning program before the TBI and bone marrow transplant. Total Body Irradiation (TBI) Techniques and equipment – The choice of a particular technique depends – the available equipment, photon beam energy, maximum possible field size, treatment distance, dose rate, patient dimensions, and the need to selectively shield certain body structures – Compensators are required to achieve dose homogeneity of l0%, excluding extremities. Total Body Irradiation (TBI) Techniques and equipment Total Body Irradiation (TBI) Techniques and equipment Total Body Irradiation (TBI) Techniques and equipment Beam energy FS = 10X10 cm, SSD= 100 cm. 15% AP/PA Lateral Total Body Irradiation (TBI) Techniques and equipment Initial Dose Build-up – Dose buildup data obtained at normal SSD (e.g., 100 cm) does not apply accurately at TBI distances (e.g., 400 cm) because of the longer distance and the intervening air – most TBI protocols do not require skin sparing – a bolus or a beam spoiler is specified to bring the surface dose to at least 90% of the prescribed TBI dose. Total Body Irradiation (TBI) Techniques and equipment Initial Dose Build-up A large spoiler screen of 1- to 2-cm-thick acrylic is sufficient to meet these requirements, provided the screen is placed as close as possible to the patient surface Total Body Irradiation (TBI) Techniques and equipment Patient Support/Positioning Devices – Important criteria include – patient comfort, stability, and reproducibility of setup and treatment geometry that allows accurate calculation and delivery of dose in accordance with the TBI protocol. Total Body Irradiation (TBI) Bilateral Total Body Irradiation Sagittal laser or floor marks to define setup distance Arms shadow the lung to provide protection Compensator s used to compensate body parts of small thickness such as head/neck, legs, etc. Total Body Irradiation (TBI) Techniques and equipment Patient Support/Positioning Devices – To achieve dose uniformity within approximately 10% along the sagittal axis of the body, compensators are designed for head and neck, lungs (if needed), and legs. – The reference thickness for compensation is the lateral diameter of the body at the level of the umbilicus – Compensators can be designed out of any material, but at the University of Minnesota they are custom-made out of aluminum. Total Body Irradiation (TBI) AP/PA Total Body Irradiation Standing position for adults Laying position for children 50% transmission block to protect the lungs, chest wall boost by electron beams Total Body Irradiation (TBI) Dosimetry data The dose calibration should be performed using the principles and methodology of AAPM TG21 The calibration is best made under geometric and phantom conditions that most nearly represent the actual treatment geometry. “dose rate to a small mass” > the value calculated from the IVSL, because of scatter contribution from the walls or the floor. This is especially true for Co-60 and lower energy linacs. In air calibrations not be performed. Total Body Irradiation (TBI) Dosimetry data Direct output calibration SAD+311cm isocente r Ion chamber measuring dose/MU using Farmer-type ion chamber placed in a water phantom of dimensions approximately 40 x 40 X 40 cm3. A table of output factors is generated as a function of depth (TMR) that can be used to calculate MU for-a patient of given at the prescription point. Total Body Irradiation (TBI) Dosimetry Data- Calculation formalism Dose rate under standard calibration conditions, e.g. 1cGy/MU Collimator setting at isocenter distance Field size equivalent to patient field size D / MU k S c (rc ) S p (rp ) TMR (d , rp ) f f '2 OAR(d ) TF Prescription depth, typically patient midline depth at the umbilicus Source to chamber distance under standard calibration conditions Source to body axis distance for TBI treatment setup Off-axis ratio for the prescription point at treatment depth d Transmission factor for the tray, spoiler screen Total Body Irradiation (TBI) Dosimetry Data- Calculation formalism D / MU k S c (rc ) S p (rp ) TMR (d , rp ) f f '2 OAR(d ) TF k. S c (rc ) S p (rp ) f f ' Dose/MU ( f , d max , rTBI ), 2 .measuring using Farmer - type ion chamber according to TG - 21 Dose/MU ( f , d max , rTBI ) IVSL Dose/MU ( f , d max , rTBI ) measurement TMR/TPR for large fields (e.g., >30 30 cm2) are not very sensitive to field dimensions. Total Body Irradiation (TBI) Dosimetry Data- Calculation formalism The TMR data obtained under standard conditions (at isocenter) must be checked for their validity at the TBI distance. In addition, the inverse square law factor must also be verified for the TBI distance. Alternatively, D/MU calculated by Equation may be compared with directly measured output factors (D/MU) at the TBI distance. Total Body Irradiation (TBI) Compensator Design Compensator design for TBI is complicated because of Large variation in body thickness, lack of complete body immobilization, and internal tissue heterogeneities. The thickness of compensator required depends on the tissue deficit material of the compensator (e.g., its density), distance of the compensator field size, and beam energy Total Body Irradiation (TBI) Compensator Design The thickness of a compensator, to at any point in the field is given by: t c TD c Where tc = compensator thickness, TD = tissue deficit, = thickness ratio ~0.7 c = compensator physical density Total Body Irradiation (TBI) Compensator Design Alternatively: I T ( AR , d R ) OARd eff t e I0 T ( A, d ) t 1 eff T ( A, d ) ln T ( AR , d R ) OARd where I0, and I are the doses administered before and after the compensator is added, T(AR,dR) and T(A,d) are the TPRs or TMRs for the reference body section and the section to be compensated for equivalent fields AR and A at midline depths dR and d, OARd is the off-axis ratio at depth d relative to the prescription point, and eff is the effective linear attenuation coefficient for the compensator material measured under TBI conditions. Total Body Irradiation (TBI) Compensator Design compensator material should be selected so that the compensator is not too bulky or of too high a density that small errors in machining would amount to large errors in dose. t c TD c pb = 11.3 g/cm3, Al = 2.7 g/cm3 Simple 1D compensators for variable body thicknesses Simple one-dimensional compensator used for lateral field irradiation technique. The compensator corrects for tissue variations along one line only. The numbers shown in this figure are direct dose measurements. The numbers in parenthesis are calculated from the entrance and exit surface measurements. AAPM report no.17 (1986) Total Body Irradiation (TBI) In-vivo Patient Dosimetry it is recommended that an in vivo dosimetry check be performed on the first 20 or so patients. TLDs surrounded by suitable buildup bolus, may be placed on the patient's skin at strategic locations and doses measured for the actual treatments given. An agreement of 5% between the calculated and measured doses is considered reasonably good. An overall dose uniformity of 10% is considered acceptable for most protocols. Total Skin Irradiation Electrons in the energy range of 2 to 9 MeV have been found useful for treating superficial lesions covering large areas of the body, such as mycosis fungoides and other cutaneous lymphomas. At these energies, electron beams are characterized by a rapid falloff in dose beyond a shallow depth and a minimal x-ray background (1% or less). Thus, superficial skin lesions extending to about 1 cm depth can be effectively treated without exceeding bone marrow tolerance. Total Skin Irradiation Translational technique: The patient lies on a motor-driven couch and is moved relative to a downward-directed beam at a suitable velocity Alternatively, the patient may be stationary and the radiation source translated horizontally • a 24-Ci 90Sr source, in the form of a 60-cm linear array, is used • The maximum energy of the particles emitted by 90Sr is 2.25 MeV • the effective depth of treatment in this case is only a fraction of a millimeter Total Skin Irradiation Large Field Technique can be produced by scattering electrons through wide angles and using large treatment distances The field is made uniform over the height of the patient by vertically combining multiple flields or vertical arcing. The patient is treated in a standing position with four or six fields directed from equally spaced angles for circumferential coverage of the body surface. Total Skin Irradiation Large Field Technique Field Flatness • Low-energy electron beams are considerably widened by scattering in air. a 6-MeV narrow electron beam, after passing through 4 m of air, achieves a Gaussian intensity distribution with a 50% to 50% width of approximately 1 m • A proper combination of more such fields or a continuous arc can lead to a larger uniform field, sufficient to cover a patient from head to foot Total Skin Irradiation Large Field Technique Field Flatness Combination of three beam intensity profiles along the vertical axis to obtain a resultant beam profile. The central beam is directed horizontally, whereas the others are directed at 18.5 degrees from the horizontal. is a weighting factor used in an equation developed by Holt and Perry. Total Skin Irradiation Large Field Technique Field Flatness Vertical beam profile at the treatment plane for a stationary single field and an arcing field Total Skin Irradiation Large Field Technique X-ray Contamination • a limiting factor in total skin irradiation • contributed by brermsstrahlung interactions produced in the exit window of the accelerator, scattering foil, ion chambers, beam-defining collimators, air, and the patient • The bremsstrahlung level can be minimized if the electron beam is scattered by air alone before incidence on the patient Total Skin Irradiation Large Field Technique X-ray Contamination • • In the Stanford technique the electron beam, after emerging from the accelerator window, is scattered by a mirror (0.028-inch Al), an aluminum scatterer located externally at the front of the collimator (0.037-inch Al), and about 3 m of air before incidence on the patient The x-ray contamination incident on the patient is reduced by angling the beam 10 degrees to 15 degrees above and below the horizontal. Because the x-rays produced in the scatterers at the collimators are preferentially directed along the central axes, they largely miss the patient Total Skin Irradiation X-ray contamination X-ray contamination along the beam central-axis Reduce x-ray contamination by angling the central axis away from the patient Total Skin Irradiation Field arrangement acrylic scatter plate ( 1 cm in thickness) 10~15° 10~15° Total Skin Irradiation Large Field Technique Dose Distribution • • • • For an oblique beam, the depth-dose curve and its dmax shift toward the surface a dose uniformity of ± 10% can be achieved over most of the body surface using the six-field technique, areas adjacent to surface irregularities vary substantially due to local scattering. Areas such as inner thighs and axilla, obstructed by adjacent body structures, require supplementary irradiation The total bremsstrahlung dose in the midline for the multiple field tech. is twice the level of a single field. Total Skin Irradiation Large Field Technique Modified Stanford technique • • • adopted the Stanford technique in principle without making alterations in the accelerator hardware the electron field is collimated by a special wide aperture insert attached at the end of the collimator. • Wider jaw setting and a specific electron energy, selected for high dose rate mode of operation • high dose rate mode is installed to allow an output of more than 2,000 MU/min an acrylic scatter plate ( 1 cm in thickness) in front of the patient to provide additional scatter to the electron beam Total Skin Irradiation Modified Stanford technique Dual field angle • widened in size by scattering: • 9-MeV electron beam, after transversing 4 m of air and an acrylic scatter plate, attains a Gaussian dose profile measuring a 90% to 90% isodose width of about 60 cm, cover a patient's width. • Along the height of the patient, two fields are angled such that in the composite dose distribution a 110% dose uniformity can be obtained over a length of about 200 cm. Total Skin Irradiation Modified Stanford technique Dual field angle 11° 11° Total Skin Irradiation Modified Stanford technique Calibration • • A thin window ( 0.05 g/cm2) p-p chamber is a suitable instrument for measuring depth dose distribution for the low-energy beams used for this technique • calibrated by intercomparison with a calibrated Farmer-type chamber, using a high-energy (10 MeV) electron beam The AAPM (Report No. 23) recommends that the total skin irradiation dose be measured at the calibration point located at the surface of the phantom and the horizontal axis Total Skin Irradiation Modified Stanford technique Calibration • A p-p chamber, embedded in a polystyrene phantom, is positioned to first measure the depth dose distribution along the horizontal axis for the single dual field • the depth dose distribution can also be measured by a film sandwiched in a polystyrene phantom and placed parallel to the horizontal axis Total Skin Irradiation Modified Stanford technique Calibration • The surface dose measurement is made at a depth of 0.2 mm. Suppose M is the ionization charge measured; the calibration point dose to polystyrene, (DP)Poly is, given by: =1, p-p chamber • calibration point dose to water, 1, close to the surface Total Skin Irradiation Modified Stanford technique Calibration • • The treatment skin dose, (Ds)poly, is defined by the AAPM (Report No. 23) as the mean of the surface dose along the circumference of a cylindrical polystyrene phantom 30 cm in diameter and 30 cm high that has been irradiated under the total skin irradiation conditions with all six dual fields. (Dp)Poly is the calibration point dose for the single dual field, then: B ranges between 2.5 and 3 for the Stanford-type technique Total Skin Irradiation Modified Stanford technique Calibration 30 cm 30 cm (Dp)Poly (Ds)Poly Total Skin Irradiation Modified Stanford technique Calibration • The composite depth dose distribution for the six dual fields may be determined by sandwiching a dosimetry film Total Skin Irradiation Modified Stanford technique In Vivo Dosimetry • • • • regions of extreme nonuniformity of dose on the patients skin. Excessive dose (e.g., 120-130%) can occur in areas with sharp body projections, curved surfaces, or regions of multiple field overlaps Low-dose regions occur when the skin is shielded by other parts of the body or overlying body folds. From in vivo measurements, areas receiving a significantly less dose can be identified for local boost. Total Skin Irradiation Modified Stanford technique In Vivo Dosimetry • • • If eyelids need to be treated, internal eye shields can be used, but the dose to the inside of the lids should be assessed, taking into account the electron backscatter from lead. TLD (<0.5 mm) are most often used for in vivo dosimetry. TLD may be calibrated in a polystyrene phantom using an electron beam of approximately the same mean energy as in the in vivo measurement conditions. Stereotactic Radiosurgery/Radiotherapy The term radiosurgery was coined by a neurosurgeon Lars Leksell in 1951 SRS single-fraction radiation therapy procedure treating intracranial lesions using a combination of a stereotactic apparatus and narrow multiple beams delivered through noncoplanar isocentric arcs SRT same procedure, multiple dose fractions SRS/SRT High degree of dose conformity and high accuracy of beam delivery The best achievable mechanical accuracy in terms of isocenter displacement from the defined center of target image is 0.2 mm 0.1 mm, although a maximum error of 1.0 mm is commonly accepted Types used in SRS and SRT: heavy-charged particles, Cobalt-60 Gamma rays megavoltage x-rays. SRS/SRT SRS TECHNIQUES X-RAY Knife linac-based SRS technique multiple noncoplanar arcs of circular (or dynamically shaped) beams converging on to the machine isocenter dose distribution be shaped to fit the lesion, • Blocking, shaping with MLC • changing arc angles and weights, • using more than one isocenter, • combining stationary beams SRS/SRT SRS TECHNIQUES Stereostatic frame Leksell Riechert-Mundinger Todd-Wells Brown-Robert-Wells (BRW) SRS/SRT SRS TECHNIQUES Stereostatic frame CT localizer head ring with posts and pins angiographic localizer patient-positioning mount SRS/SRT SRS TECHNIQUES Stereostatic frame angiographic localizer CT localizer DELINEATION OF BRAIN AVMs ON MR-ANGIOGRAPHY FOR THEPURPOSE OF STEREOTACTIC RADIOSURGERY DENNIS R. BUIS et al. Int. J. Radiation Oncology Biol. Phys., Vol. 67, No. 1, pp. 308–316, 2007 SRS/SRT SRS TECHNIQUES The CT localizer frame is equipped with nine fiducial rods, which appear as dots in the transaxial slice image any point in the image can be defined in terms of the frame coordinates. SRS/SRT SRS TECHNIQUES patient docking device couples the frame to the accelerator through the patient support system (pedestal or couch-mount bracket) SRS/SRT SRS TECHNIQUES The magnetic resonance imaging (MRI) localizer is a slightly modified version of the CT localizer and is compatible with MRI. SRS/SRT SRS TECHNIQUES A special relocatable head ring, Gill-Thomas-Cosman (GTC), has been designed for fractionated SRT uses a bite block system, SRS/SRT SRS TECHNIQUES Linac isocentric accuracy An essential element of the SRS procedure is the alignment of stereotactic frame coordinates with the linac isocenter Acceptable specification of linac isocentric accuracy within a sphere of radius 1.0 mm with any combination of gantry, collimator, and couch rotation. SRS/SRT Stereotactic accuracy using the phantom base to check the alignment of radiation isocenter with the target point defined by the coordinates set on the BRW pedestal. SRS/SRT Overall accuracy Before the SRS system is declared ready for patient treatments, the entire radiosurgery procedure should be tested for geometric accuracy This can be accomplished by using a suitable head phantom The comparison of these coordinates with the known coordinates of these points in the phantom gives the geometric accuracy. localization error, LE SRS/SRT Beam collimation SRS or SRT is normally used for small lesions requiring much smaller fields the geometric penumbra (inversely proportional to SDD) must be as small as possible attachment of long cones below the x-ray jaws extends the SDD, thus reducing the geometric penumbra. SRS/SRT Beam collimation SRS fields can be shaped with cone or MLC SRS/SRT Gamma knife 201 Co-60 sources are housed in a hemispherical shield Source to focus distance of 40.3 cm The central axes of all 201 beams intersect at the focus with a mechanical precision of 0.3 mm. SRS/SRT Gamma knife 照野孔大小分別為直 徑 4, 8, 14 及 18 mm 4 組不同大小照野孔的 準直頭盔 治療時固定於病患頭部的立體定位頭架與治療床定位架接合,準 直頭盔再與治療床外部固定結構接合 SRS/SRT Gamma knife Selected channels can be blocked with plugs to shield the eyes or to optimize the dose distribution. The plugs are made of 6-cm-thick tungsten alloy Stereotactic CT, MRI, or angiography can be used for target determination Most users of Gamma Knife technology have restricted lesion size to a mean spherical diameter of 35 mm (and usually less) The accuracy of dose delivery using the Gamma Knife as tested at the University of Pittsburgh was found to be approximately 0.25 mm SRS/SRT Dosimetry Three quantities of interest: • central axis depth distribution (%dd or TMR), • cross-beam profiles (off-axis ratios), • output factors (Sc,p or dose/MU) Measurement of these quantities is complicated by two factors: • detector size relative to the fieId dimensions • a possible lack of charged particle equilibrium • the sensitive volume of the detector must be irradiated with uniform electron fluence (e.g., within 0.5%). SRS/SRT Dosimetry Types of detector systems have been used in SRS dosimetry: • ion chambers, film, TLD, and diodes ion chamber is the most precise and the least energydependent system but usually has a size limitation; film has the best spatial resolution but shows energy dependence and a greater statistical uncertainty (e.g., 3%); TLD show little energy dependence and can have a small size but suffer from greater statistical uncertainty as the film; diodes have small size but show energy dependence as well as possible directional dependence SRS/SRT Dosimetry Cross-beam profiles • a detector size of 3.5 mm diameter, circular fields in the range of 12.5 to 30.0 mm in diameter can be measured accurately within 1 mm. • little change in the photon energy spectrum across small fields, diodes and film are the detectors of choice AAPM Report No. 54 Cross-beam profiles 3x3,CC13 VS TPS 3x3, CC01, Film VS TPS 2x2, Film VS TPS 1x1, Film VS TPS 69 SRS/SRT Dosimetry Depth dose distribution • For field sizes of diameter 12.5 mm or greater, it has been shown that the central axis depth dose can be measured correctly with a p-p ionization chamber of diameter not exceeding 3.0 mm • Film or diodes can also be used for central axis depth dose distribution, especially for very small field sizes SRS/SRT Dosimetry Output factors 1.2 1.0 0.8 ROF 0.6 0.4 0.2 ROF-TPS ROF-Pinpoint ROF-Semiflex ROF-Farmer ROF-5 mm ROF-2 mm ROF-1 mm ROF-0.5 mm 0.0 0.0 1.0 2.0 3.0 4.0 5.0 FS (cm) 6.0 7.0 8.0 9.0 10.0 SRS/SRT Dosimetry Output factors for fields of diameter 12.5 mm and larger, clindrical or p-p chambers of 3.5 mm diameter allow the output factors to be measured accurately to within 0.5% Stereotactic body radiation therapy References: 1. Stereotactic body radiation therapy: The report of AAPM Task Group 101. Med. Phys. 37 (8), August 2010, 4078-4101. 2. Technical Basis of Radiation Therapy, Practical Clinical Applications. Fifth Edition Introduction SBRT refers to an emerging radiotherapy procedure that is highly effective in controlling early stage primary and oligometastatic cancers at locations throughout the abdominopelvic and thoracic cavities, and at spinal and paraspinal sites. Introduction The major feature that separates SBRT from conventional radiation treatment ◦ large doses in a few fractions, which results in a high biological effective dose (BED) In order to minimize the normal tissue toxicity, conformation of high doses to the target and rapid fall-off doses away from the target is critical. Introduction In SBRT, confidence in this accuracy is accomplished by the integration of ◦ modern imaging, ◦ simulation, ◦ treatment planning, ◦ delivery technologies into all phases of the treatment process; from treatment simulation and planning, and continuing throughout beam delivery. Introduction History and rationale for SBRT Over 4000 publications have affirmed the clinical usefulness of SRS in the treatment of benign and malignant lesions, as well as functional disorders. The radiobiological rationale for SBRT is similar to that for SRS. The clinical outcomes of SBRT for both primary and metastatic diseases compare favorably to surgery with minimal adverse effects. Current status of SBRT-patient selection criteria The majority of patients treated with SBRT ◦ lung, liver, and spinal tumors. Most investigators limit eligibility to ◦ well-circumscribed tumors ◦ maximum cross sectional diameter of up to 5 cm. The use of SBRT as a boost in addition to regional nodal irradiation. Current status of SBRT-patient selection criteria assessment of patient eligibility should include a careful evaluation of normal tissue function and dose distribution. Typically, pulmonary function and the volume of normal liver that is irradiated are the most immediate considerations. Tumors proximal to mainstem bronchi, trachea, esophagus, gastric wall, bowel, blood vessels, or spinal cord should be approached with great caution. Simulation imaging SBRT requires precise delineation of patient anatomy, targets for planning, and clear visualization for localization during treatment delivery. ◦ CT or 4DCT for visualizations and dose calculation ◦ MRI and PET images assist in target and visualization ◦ Dynamic contrast-enhanced CT is the most sensitive study for the hepatic system Simulation imaging Recommendation: ◦ A typical scan length should extend at least 5–10 cm superior and inferior beyond the treatment field borders. ◦ For noncoplanar treatment techniques, the scan length may further be extended by 15 cm inferior/superior beyond the target borders Simulation imaging Recommendation: ◦ all OAR should be included and covered by the selected scan length so they can be considered by the TPS and evaluated with DVH ◦ tomographic slice thickness of 1–3 mm though the tumor site is recommended Abdominal compression plate Activ e breath control (ABC) JOURNAL OF APPLIED CLINICAL MEDICAL PHYSICS, VOLUME 14, NUMBER 6, 2013 Evaluation of two synchronized external surrogates for 4D CT sorting Carri K. Glide-Hurst,a Megan Schwenker Smith, Munther Ajlouni, Indrin J. Chetty Data acquisition for mobile tumors Techniques to image moving targets include ◦ slow CT, breath-hold techniques, gated approaches, 4DCT used in conjunction with maximum-intensity projection, respiration-correlated PET-CT Imaging artifacts slower acquisitions to characterize the movement of the target can also lead to motion artifacts Motion-related artifacts may be improved by immobilization and patient cooperation. Imaging artifacts a spherical object (R=1.2 cm), CT scanned while periodically moving on a sliding table (A=1 cm, T=4.4 s). different artifacts obtained by standard axial CT scanning. imaged with 4DCT Four-dimensional computed tomography: Image formation and clinical protocol Eike Rietzel, et al. Med. Phys. 32 (4), April 2005 Imaging artifacts Recommendation: ◦ If target and radiosensitive critical structures cannot be localized on a sectional imaging modality with sufficient accuracy because of motion and/or metal artifacts, SBRT should not be pursued as a treatment option Gated CT (phase-50% ) MIP CT Treatment planning prescribing dose for SBRT ◦ A limited volume of tissue, containing the gross tumor and its close vicinity, is targeted for treatment through exposure to a very high dose per fraction, and hotspots within the target are often deemed to be acceptable. Treatment planning prescribing dose for SBRT ◦ The volume of normal tissue receiving high doses outside the target should be minimized. Dose fall-off outside the target should be sharp. Treatment planning In SBRT (especially for metastatic lung, liver, and paraspinal cases), GTV = CTV Typical SBRT margins for defining the minimal distance separating the CTV and PTV surfaces are 0.5 cm in the axial planes and 1.0 cm in the inferior/superior directions for treatments that were performed in conditions that suppressed respiratory motion. Treatment planning Dose prescriptions in SBRT are often specified at low isodoses (e.g., 80% isodose) and with small or no margins for beam penumbra at the target edge Hot spots within the target volumes are generally viewed to be clinically desirable, as long as there is no spillage into normal tissue. Treatment planning parameters that affect the dose fall-off ◦ multiple nonoverlapping beams ◦ beam energy. A 6 MV photon beam provides a reasonable compromise between the beam penetration and penumbra characteristics for SBRT lung applications. ◦ the resolution of beam shaping (e.g. MLC leaf width). The 5 mm MLC leaf width has been found to be adequate for most applications, with negligible improvements using the 3 mm leaf width MLC for all but the smallest lesions (< 3 cm in diameter). Beam selection and beam geometry the avoidance of sensitive organs, mechanical constraints imposed by the equipment and short beam paths In general, a greater number of beams yields better target dose conformity and dose fall-off away from the target. ◦ limit the number of beams or arcs ◦ avoiding beam overlaps Calculation grid size The calculation grid resolution used in the TPS affects the accuracy of the dose distribution calculated. a 2.5 mm isotropic grid produces an accuracy of about 1% in the high-dose region of an IMRT plan consisting of multiple fields. an accuracy of 5% for an isotropic grid resolution of 4 mm Calculation grid size dose difference of 2.3% of the prescribed dose for 2 mm calculation grids as compared to 1.5 mm grids, rising to 5.6% for 4 mm grids. Recommendation: ◦ The use of an isotropic grid size of 2 mm or finer. ◦ The use of grid sizes greater than 3 mm is discouraged for SBRT. Bioeffect-based treatment planning SBRT involves the application of high fractional doses in a range not studied in prior decades. To evaluate the possible biological effect of a SBRT treatment plan ◦ BED concept, ◦ the normalized total dose (NTD) concept, ◦ the equivalent uniform dose (EUD) concept. Normal tissue dose tolerance Normal tissue dose limits for SBRT are still quite immature. should not be directly extrapolated from conventional radiotherapy data. radiobiological factors, particular attention should be paid ◦ fraction size, ◦ Total dose, ◦ time between fractions, ◦ overall treatment time, Normal tissue dose tolerance Normal tissue dose tolerance Because of the sparseness of long-term follow-up for SBRT, it should be recognized that the data in both Table III and the published reports represent, at best, a first approximation of normal tissue tolerance. Localization, tumor-tracking, and gating techniques for respiratory motion management Image-guided techniques ◦ Fluoroscopy ◦ gated radiographs ◦ cone beam imaging of soft tissue Cone beam scans can have an acquisition time 60 s or more, capturing the average tumor position over 15 or more breathing cycles, which may correspond well to the planning ITV as obtained from 4DCT. Respiratory gating techniques dose is delivered only in particular phases of the respiratory cycle with the goal of reducing the probability of delivering dose to normal tissue and underdosing the target Respiratory gating increases treatment time as compared to nongated treatments; published duty cycles (ratio of beam on to total beam delivery time) range from 30% to 50% the benefit of gated beam delivery is minimal with motion amplitudes smaller than 2 cm Special dosimetry considerations Heterogeneity correction becomes extremely important in situations where the target is surrounded by low-density tissue such as the lungs. Pencil-beam algorithms accounting for only 1D scatter corrections are not recommended for accurate estimate of the dose in such tumors and in general for any lung tumors convolution/superposition perform adequately in most clinical situations Thank you for your attention!