* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Alcohols
Survey
Document related concepts
Transcript
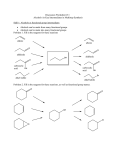
ALCOHOLS ALCOHOLS • ALCOHOLS ARE A FAMILY OF ORGANIC COMPOUNDS THAT HAVE A HYDROXYL (-OH) GROUP ATTACHED TO A CARBON ATOM. • ALCOHOLS ARE VERY DIFFERENT FROM INORGANIC BASES. IN ALCOHOLS, THE HYDROXYL GROUP IS ATTACHED TO A CARBON BY A COVALENT BOND; THIS IS DIFFERENT FROM THE HYDROXIDE ION (OH-) WHICH IS A NEGATIVELY CHARGED PARTICLE FOUND IN BASES. IN BASES, THE HYDROXIDE ION IS HELD TO A POSITIVE ION BY AN A IONIC BOND. • ALCOHOLS HAVE THE GENERAL FORMULA CnH 2n+ 1OH CLASSIFICATION OF ALCOHOLS • ALCOHOLS ARE CLASSIFIED AS PRIMARY, SECONDARY OR TERTIARY, DEPENDING ON WHETHER THE CARBON THAT HAS THE –OH GROUP IS BONDED TO ONE, TWO OR THREE OTHER CARBONS, RESPECTIVELY. PRIMARY SECONDARY TERTIARY NAMING ALCOHOLS 1. NAME THE PARENT COMPOUND. FIND THE LONGEST CHAIN THAT HAS THE HYDROXYL SUBSTITUENT ATTACHED. NAME THE CHAIN ACCORDING TO THE NUMBER OF CARBON ATOMS AND CHANGE THE ENDING TO “OL”. 2. NUMBER THE CARBON ATOMS N THE MAIN CHAIN, STARTING AT THE END NEARER THE HYDROXYL GROUP. 3. WRITE THE NAME BY PLACING THE NUMBER THAT LOCATES THE HYDROXYL GROUP IMMEDIATELY BEFORE THE PARENT COMPOUND NAME. 4. WRITE THE NAMES OF ANY SUBSTITUENTS AND THEIR POSITIONS, LISTING THEM ALPHABETICALLY. ALCOHOLS • IDENTIFY THE FOLLOWING COMPOUNDS AS PRIMARY, SECONDARY OR TERTIARY. ALCOHOLS • NAME THE FOLLOWING COMPOUNDS: ALCOHOLS • WRITE THE STRUCTURAL FORMULA FOR THE FOLLOWING COMPOUNDS 4-METHYL-4-HEPTANOL 3-ETHYL-4-METHYL-2-HEXANOL PROPERTIES OF ALCOHOLS • ALCOHOLS ARE MUCH MORE POLAR THAN HYDROCARBONS BECAUSE OF THE ELECTRONEGATIVE OXYGEN ATOM IN THE –OH GROUP. • BECAUSE OF THIS POLARITY, HYDROGEN BONDING OCCURS IN ALCOHOLS AND BECAUSE OF THE STRONGER INTERMOLECULAR FORCES, ALCOHOLS HAVE HIGHER BOILING POINTS THAN THEIR RELATED ALKANES. • BECAUSE OF THE POLAR –OH GROUP, ALCOHOLS CAN FORM HYDROGEN BONDS WITH WATER. AS A RESULT, ALCOHOLS ARE MORE SOLUBLE IN WATER THAN ALKANES OF COMPARABLE MOLAR MASS. FOR EXAMPLE, ETHANOL, THE ALCOHOL PRESENT IN ALCOHOLIC DRINKS, IS SOLUBLE IN WATER. HOWEVER, AS THE HYDROCARBON CHAIN BECOMES LONGER, THE SOLUBILITY OF ALCOHOLS IN WATER DECREASES. REACTIONS OF ALCOHOLS- COMBUSTION REACTIONS OF ALCOHOLS-OXIDATION OXIDATION • ALCOHOLS CAN REACT WITH OXIDIZING AGENTS SUCH AS POTASSIUM PERMANGANATE OR POTASSIUM DICHROMATE. • WHEN WRITING THESE REACTIONS, THE OXIDIZING AGENT IS SHOWN AS +[O] ABOVE THE ARROW. • THE PRODUCT OBTAINED WILL DEPEND ON WHETHER THE ALCOHOL IS PRIMARY, SECONDARY, OR TERTIARY. OXIDATION-PRIMARY ALCOHOLS PRIMARY ALCOHOLS ARE OXIDIZED IN A TWO STEP REACTION. 1. MILD OXIDATION YIELDS AN ALDEHYDE. 2. FURTHER OXIDATION YIELDS A CARBOXYLIC ACID. THE H FROM THE –OH AND AN H FROM THE CARBON BEARING THE –OH ARE REMOVED. CONSEQUENTLY, A DOUBLE BOND FORMS BETWEEN THE CARBON AND THE OXYGEN. OXIDATION OF PRIMARY ALCOHOLS OXIDATION OF PRIMARY ALCOHOLS OXIDATION OF PRIMARY ALCOHOLS OXIDATION OF PRIMARY ALCOHOLS • REMEMBER: OXIDATION OF SECONDARY ALCOHOLS • OXIDATION OF SECONDARY ALCOHOLS YIELDS A KETONE. • KETONES CAN NOT BE FURTHER OXIDIZED. • AS IN PRIMARY ALCOHOLS, THE H FROM THE –OH AND AN H FROM THE CARBON BEARING THE –OH ARE REMOVED, AND A DOUBLE BOND FORMS BETWEEN THE CARBON AND OXYGEN ATOMS. OXIDATION OF TERTIARY ALCOHOLS • TERTIARY ALCOHOLS DO NOT OXIDIZE. OXIDATION OF ALCOHOLS • POTASSIUM DICHROMATE IS AN ORANGE SOLUTION. AS THE ALCOHOL IS OXIDIZED, THE CHROMIUM BECOMES REDUCED AND THE SOLUTION TURNS GREEN. • HOWEVER, IN TERTIARY ALCOHOLS, SINCE THERE IS NO OXIDATION, THE SOLUTION STAYS ORANGE. OXIDATION OF ALCOHOLS