* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Genetics of a wing size difference between two Nasonia species
Survey
Document related concepts
Transcript
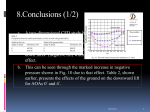
Genetics of a wing size difference between two Nasonia species R. F. WESTON, I. QURESHI & J. H. WERREN Biology Department, University of Rochester, Rochester, NY 14627, USA Keywords: Abstract evolutionary genetics; Nasonia; parasitic wasps; speciation; wing size. Very little is known about the genetics of morphological differences between species. This study investigates the genetic basis of a signi®cant morphological difference between males of two closely related species of the parasitoid wasp Nasonia. One of the de®ning characters of species in the genus Nasonia is male forewing size. The forewings of Nasonia giraulti males are 2.4 times larger than the forewings of Nasonia vitripennis males. Genetic analysis of hybrids between these species indicates that this difference is due to the effect of a few genes. Also discussed is the possible role of `pseudo linkage' in analysis of F2 hybrids. Pseudo linkage occurs when genes affecting a trait are linked to interacting hybrid lethal loci, and can lead to an overestimation of the number of regions involved in a phenotype. The large wing trait of N. giraulti was introgressed into a N. vitripennis background. Analysis of this introgression line indicates that 44% of the difference in wing size between the species is due to the presence of a single gene, or a few tightly linked genes, located on linkage group IV. Furthermore, the introgressed region appears to affect the width of the wing more strongly than the length. Indirect results suggest that this region affects wing cell size, rather than cell number. Results are consistent with the view that morphological and adaptive differences between species can have a simple genetic basis. Introduction Over the past century there have been various studies on the genetic basis of morphological differences within and between species (see reviews by Lande, 1981; Orr & Coyne, 1992). These studies have examined the genetic architecture of morphological traits in domesticated strains of plants and animals and their wild relatives, strains that differ because of arti®cial selection in the laboratory; and species or subspecies that differ morphologically, presumably because of differing natural and sexual selective pressures. Mutations of large effect are common in animal and plant breeding (Gottlieb, 1984; Piper & Shrimpton, 1989) but arti®cial selection experiments in the laboratory have resulted in responses due to mutations of both large effect (Crow, 1957) and small Correspondence: John H. Werren, Biology Department, University of Rochester, Rochester, NY 14627, USA. Tel: 716 275 3889; fax: 716 275 2070; e-mail: werr@uhura.cc.rochester.edu 586 effect (Crow, 1957; Lande, 1981; Shrimpton & Robertson, 1988). In disturbed natural populations, however, adaptations are commonly based on mutations with large effects (Lees, 1981; McNair, 1981). Less common are genetic analyses of morphological differences between natural populations or closely related species. Examples include colour differences in Lepidoptera (Clarke & Sheppard, 1959; Sheppard, 1962; Turner, 1981) and newts (Spurway, 1953), ¯oral traits in closely related species of monkey¯owers (Bradshaw et al., 1995) and morphological differences between sympatric species of sticklebacks (Hat®eld, 1997). Genes of large effect have been found in ¯oral character differences between closely related monkey ¯owers (Bradshaw et al., 1995) and some morphological characters of stickleback species (Hat®eld, 1997). However, differences in genital arch morphology between closely related Drosophila species are due to many genes of small effects (Coyne et al., 1991; Liu et al., 1996). A dif®culty in studying morphological differences between closely related species is that typically the differences are small J. EVOL. BIOL. 12 (1999) 586±595 ã 1999 BLACKWELL SCIENCE LTD Genetics of species wing size differences or subtle, or, in cases of signi®cant morphological divergence, the species cannot be crossed or lack availability of techniques making them suitable for genetic analyses. The purpose of this study is to investigate the genetic basis of a large wing size difference between males of two closely related species of the parasitoid wasp Nasonia. The genus Nasonia consists of three parasitic wasp species: N. vitripennis (Nv), N. giraulti (Ng) and N. longicornis (Nl) (Darling & Werren, 1990). N. vitripennis is a cosmopolitan species while N. longicornis is found only in the western United States, and N. giraulti is found in the north-east United States (Darling & Werren, 1990). One of the primary morphological differences between the species is male forewing size. N. vitripennis males have short, narrow vestigial wings and the males are incapable of ¯ight. Ng wings are »1.35 times longer than and 1.89 times wider than Nv. N. longicornis (Nl) males have intermediate sized wings (»1.2 times longer and 1.5 times wider than Nv). Preliminary experiments indicate that N. giraulti and N. longicornis males are capable of limited ¯ight. The three species will mate with one another and, once cured of their associated cytoplasmic bacterial infections (Wolbachia), form viable and fertile hybrids (Breeuwer & Werren, 1990, 1995). Due to the haplodiploid sex determination in Nasonia, F1 hybrids produced in interspeci®c crosses are female, only males are derived from unfertilized eggs and therefore have the genotype of the maternal species. Virgin F1 hybrid females produce all male (haploid) progeny. While partial F2 hybrid breakdown occurs, survival rates are suf®cient to allow backcrossing and introgression of genetic regions between the species. The ability to perform interspeci®c crosses within Nasonia, availability of genetic markers and existence of haplodiploid sex determination facilitates our analysis of the genetic basis of forewing size differences between the species. By analysing F2 recombinant hybrid males from these crosses we can determine whether the Nasonia male wing size difference is due to many genes of minor effect or to a few genes of large effect. In addition, we are able to introgress genes involved in the wing size difference from one species into the genetic background of the other. These techniques have allowed us to determine that »44% of the difference in the average normalized wing multiples between the species is due to the presence of a single gene, or a region containing tightly linked genes. Additionally, it appears that the region affects wing width more strongly than wing length, causing a 26% increase in wing width but only a 17% increase in wing length. Materials and methods The general biology of Nasonia has been described by Whiting (1967). Nasonia are ectoparasitoids of a number of calliphorid ¯ies found in bird nests and carcasses. All 587 experiments were performed at 25 °C on Sarcophaga bullata with constant light. Under these conditions the generation time of Nasonia is »14 days. Nasonia stocks A number of strains antibiotically cured of cytoplasmic incompatibility micro-organisms (Wolbachia) were used for these experiments. Antibiotically cured strains are used because elimination of the Wolbachia permits formation of F1 hybrids (Breeuwer & Werren, 1990). AsymC is an antibiotically cured strain of the N. vitripennis wild-type strain (LabII). For the purpose of this paper AsymC is designated as Nv. Similarly, an antibiotically cured strain of N. giraulti (RV2T) is designated as Ng[G], where the G in brackets indicates the strain has a N. giraulti cytotype. The cytotype refers to heritable cytoplasmic factors, such as mitochondria, found in the cytoplasm of an individual. A third strain (R16A) is an introgression stock, created by 16 generations of backcrossing, of the N. giraulti nuclear complement into a N. vitripennis cytotype (see Breeuwer & Werren, 1995, for a description). This strain is designated Ng[V]. N. vitripennis has ®ve chromosomes and is characterized by ®ve linkage groups (Whiting, 1967). In addition, ®ve antibiotically cured N. vitripennis mutant eye strains (corresponding to the ®ve linkage groups) were used to determine linkage of the N. giraulti wing type: RED833R (linkage group I), RDH5R (linkage group II), BK424tet (linkage group III), OR123R (linkage group IV) and ST318tet (linkage group V). Wing measurements Forewings were removed from the body of male wasps and both bodies and wings were mounted on a slide covered with double-sided cellophane tape. Measurements were taken using a dissecting scope at 25´ magni®cation and ocular micrometer. The length of the wing is de®ned as the distance from its distal end to the mass of dark connective tissue at the proximal. Only the transparent portion of the wing was included in the length measurement. The width of the wing was measured at its widest part, perpendicular to the length measurement. In addition to the wing measurements, the width of the head between the eyes in the region just anterior to the ocelli was measured (referred to as head width). Head width has been shown to be a good approximation for relative body size and fecundity (Skinner, 1983). The bristle density in a region of the wing was also measured. Bristle density was approximated by counting the number of bristles in a 0.0156-mm2 grid. This grid was positioned just below the end of the stigmal vein and every bristle with its base within the grid was counted, including those on the underlying side of the wing. J. EVOL. BIOL. 12 (1999) 586±595 ã 1999 BLACKWELL SCIENCE LTD 588 R . F. W E S T O N E T A L . Transformations We used a `normalized wing multiple' to characterize wing sizes. The `wing multiple' was calculated by multiplying the width of a wing by its length. However, wing size increases allometrically with body size in both species (Nv P < 0.001, Ng P < 0.001, Linear Regression). Therefore, the wing multiple was normalized for body size by dividing it by the measured head width of each wasp. The normalized wing multiple eliminates most of the correlation between wing size and body size in both species (n 75, P > 0.50 for Nv, n 113, P > 0.5 for Ng[V], Linear Regression). Accuracy in measurements To check the accuracy and reproducibility of the wing measurements, measurements of the Nv parental strain were repeated a month after their initial measuring. These measurements were performed without any knowledge of the original measurements. Comparison of the second measurement to the ®rst showed an average 0.4 1.8SD difference in wing multiples (n 49). This difference is small compared to the mean normalized wing multiple of Nv (20.1 2.1SD, n 49, 1.9% difference) or to the difference between Nv and Ng (29, 1.3% difference). F2 hybrid crosses For all interspeci®c crosses, males and females were collected as virgin pupae and allowed to eclose. After eclosion, females were mass mated to males (3 males ´ 10 females) or left as virgins. Because Nasonia are haplodiploid, virgin females produce only male progeny by parthenogenesis. After 24 h, males were removed (in mated crosses), and females were transferred to individual vials and provided with two hosts. Females were transferred to fresh hosts after 2 days. To produce F2 hybrid strains for linkage analysis, males of all the N. vitripennis strains (AsymC, RED833R, RDH5R, BK424tet, OR123R, ST318tet) were crossed to Ng[V] females. After 11 days, hosts were opened and wasp pupae sexed. The F1 female hybrids were collected as virgins and allowed to eclose. Adult F1 hybrid virgins were set singly on two hosts to obtain F2 recombinant hybrid males, who were scored for wing phenotype. Introgression line (INTW1) The INTW1 stock was created by crossing N. vitripennis females to N. giraulti males, and then backcrossing F1 females to N. vitripennis males. In each subsequent generation, virgin females from families also containing some large-winged males were crossed to N. vitripennis, thus selecting for the Ng large-wing trait while introgressing into an Nv background. These backcrosses were J. EVOL. BIOL. 12 (1999) 586±595 ã 1999 BLACKWELL SCIENCE LTD carried out for ®ve generations, in principle reducing the N. giraulti genome to 3.1% of the total genome. This resulted in the INTW1 line which is almost completely N. vitripennis in genome, contains Nv cytotype, and has a male wing size intermediate to Nv and Ng. After ®ve generations of introgression, the INTW1 strain was maintained heterozygously for several generations, then large winged males were crossed to Nv females and the progeny were maintained into diapause. These were removed from diapause in April 1996 and purebred for the large wing trait. Purebred Wg lines After removal from diapause, virgin INTW1 females were mated to Nv males. To produce a pure-bred large winged line, virgin daughters of mothers producing large winged sons were mated to large winged males. This was repeated each generation until we had produced families homozygous for the Ng large wing trait (Wg). During the initial crosses of Wg/Wg females to Wg males, ®ve isofemale sublines were created. A single line, IWTW1.1, was used for subsequent studies. To determine the linkage of the large-wing trait, INTW1.1 males were mated singly to mutant females of each eye marker. Males were mated to a mutant female and then transferred to a female carrying a different mutant marker. These females were individually given two hosts and their F1 female pupae were collected and allowed to eclose. Adult F1 virgin females were provided with two hosts and their male progeny were scored for segregation of the N. giraulti wing trait. Estimating the number of genes involved in wing size The minimum number of effective factors was calculated using the Wright±Lande equation adapted for a haplodiploid system (eqn 1) (Orr and Jones, personal communication). Where ne is the minimum number of effective loci involved in the trait, P1 and P2 are the parental means for trait A, and rA is the variance in trait A among the progeny. This provided an estimate of the minimum number of genes involved in wing size determination between Nv and Ng: ne P2 ÿ P1 2=4 r2A : 1 Statistics Student's t-tests were used to determine whether differences in wing, head and bristle measurements between strains were statistically signi®cant. For these tests a comparison of two means was used in order to determine the probability that the mean normalized wing multiples of the mutant and wild-type F2 males were different from one another. To determine whether two nonparametric distributions differed in their dispersion around the Genetics of species wing size differences 589 median, a Mann±Whitney U-test was performed on the absolute values of the difference between the medians and observed data. We refer to this test as Deviation From the Median (DFM). Results Characterization of Nv and Ng species wing size differences Measurements of length, width and head size of the wild type strains of Nv and Ng[G] were performed. Head size does not vary signi®cantly between the species (Nv 9.2 0.8SD, n 75, Ng 9.4 0.7SD, n 83) (P > 0.05). Analysis of wings revealed wing lengths between the species overlap slightly, but wing widths do not overlap. The average measurements are provided in Table 1. Both length (P < 0.001) and width (P < 0.001) differ signi®cantly between the species. The same results were found after normalizing the length and width measurements with respect to overall wasp body size. After transforming the measurements to a normalized wing multiple, which is a rough estimate of wing area normalized to body size, the N. vitripennis (Nv) and N. giraulti (Ng) distributions proved to be completely nonoverlapping (Fig. 3) and mean values are statistically different (P < 0.001 Fig. 1). Additionally the wild type Ng[G] was compared to the Ng[V] strain, which possesses a N. giraulti nuclear genome and a N. vitripennis cytotype. No signi®cant differences were found between male wing size in these strains. Previous work in Drosophila shows that the number of wing bristles can provide an estimate of the number of cells in the wings (Dobzhansky, 1929). If a similar pattern occurs in Nasonia (not yet established) the bristle density could be used to estimate cell size in wings. The wing bristle density characteristic of each species was determined. Bristle density in Nv (133 22SD, n 48) is approximately three times higher than in Ng[V] (46 7SD, n 75) (P < 0.01). The lower bristle density suggests N. giraulti has larger wing cells than in N. vitripennis (Fig. 2). Table 1 Basic characterization of the strains. The mean and standard deviation of each measurement is given. Measurements are in ocular units (1 unit = 0.2 mm). Fig. 1 The distribution of normalized wing multiples in Nv ´ Ng[V] F2 males. (a) The normalized wing multiples of male forewings from the Nv and Ng[V] parental strains. (b) The initial cross of a Nv male to a Ng[V] female shows a nonunimodal distribution. F2 hybrids If the difference in male wing size between N. vitripennis and N. giraulti is due to the presence of many genes of small effect, a unimodal and normal distribution of wing size is expected among the F2 males. Alternatively, if the difference is due primarily to a gene of large effect, a bimodal distribution is expected as the gene causing a major phenotypic effect is received by only half of the progeny. Figure 1 shows the distribution of normalized Strain Wing width Wing length Parentals Nv N1 Ng[G] Ng[V] 7.3 0.7 10.9 0.6 13.9 1.4 14.0 0.9 25.3 29.6 34.2 34.5 Introgression lines INTW1.1 INTW1.2 INTW1.3 10.0 0.8 10.0 0.5 9.6 0.6 30.3 1.7 30.3 1.4 29.8 3.8 Head width 2.0 1.5 2.9 2.3 9.2 9.4 9.4 9.9 0.8 0.5 0.7 0.7 9.2 0.7 9.2 0.4 9.0 0.5 Normalized wing multiple n 20.1 34.3 50.5 49.1 2.3 2.5 6.1 4.5 75 25 83 113 32.9 3.1 33.2 2.8 31.8 4.6 105 40 40 J. EVOL. BIOL. 12 (1999) 586±595 ã 1999 BLACKWELL SCIENCE LTD 590 R . F. W E S T O N E T A L . Table 2 Linkage analysis of the Wg wing trait. The mean normalized wing multiple and standard deviation of the F2 males resulting from all crosses are shown (male/female). Signi®cance is based on the results of Student's t-tests comparing the mean of normalized wing multiples of mutant F2 males to the mean of normalized wing multiples among wild-type F2 males. Measurements are in ocular units (1 unit = 0.2 mm). Fig. 2 The number of bristles in a region of the wing next to the stigmal vein. Bristle number was collected for the INTW1 and parental lines and plotted against their normalized wing multiples. Bristle density clearly decreases as wing size increases. wing multiples among the recombinant F2 males produced when a Nv male is crossed to a Ng[V] female (n 124). The distribution is clearly not normal (v2 18.2, P < 0.001; d.f. 6) and appears to be bimodal, suggesting that there is a major gene affecting wing size. The dispersion observed in the data around the median (DFM) is lower in the F2 hybrids than in the pooled parental population (P < 0.001). The inward shifting of the distribution indicates either the presence of small effect modi®ers as well as the presence of genes of major effect, or alternatively maternal effects of the F1 hybrid genotype. Using the modi®ed Wright±Lande equation (see Methods) we calculate the minimum number of genes involved in male wing size to be 1.6. This calculation assumes additivity of gene effects. Epistatic interactions, which can also generate bimodal distributions, could invalidate this estimate. To further investigate the genetics of the wing size difference, we performed a linkage analysis of the wing trait by crossing strains of N. vitripennis carrying various mutant eye markers, to N. giraulti. This allows us to determine which linkage group has the largest effect on wing size. Analysis of the F2 recombinant hybrid males of these crosses (Table 2) shows the N. vitripennis wing type segregates strongly with the mutant eye marker OR123, located on linkage group IV (OR eye F2 males 25.2 6.3SD, n 62; WT eye F2 males 36.7 6.4SD, n 62). The N. vitripennis wing also shows strong segregation with RED833 (linkage group I) and weaker segregation with ST318 (linkage group V) but not with RDH5 (linkage group II) or BK424 (linkage group III) (Table 2). J. EVOL. BIOL. 12 (1999) 586±595 ã 1999 BLACKWELL SCIENCE LTD Mutant Wild-type Overall Parentals Nv Ng[V] INTW1.1 ± ± ± ± ± ± 20.1 2.3 49.1 4.5 32.9 3.1 F2 hybrid crosses Nv/Ng[V] RED833R/Ng[V] RDH5R/Ng[V] BK424tet/Ng[V] OR123R/Ng[V] ST318tet/Ng[V] ± 25.1 30.5 30.4 25.2 26.0 7.8 7.8 7.9 6.3 6.5 ± 34.2 30.2 30.8 36.2 33.4 8.8 7.5 8.9 7.1 8.4 30.3 29.7 30.3 30.6 30.7 29.5 7.4 9.5 7.6 8.2 8.7 8.3 F2 introgression crosses INTW1.1/Nv INTW1.1/RED833R INTW1.1/RDH5R INTW1.1/BK424tet INTW1.1/OR123R INTW1.1/ST318tet ± 26.4 26.2 26.0 20.3 26.0 7.7 6.7 7.4 2.0 6.5 ± 24.7 25.7 26.1 31.9 26.2 6.7 7.3 5.9 3.4 6.2 28.2 25.6 25.9 26.0 26.1 26.1 5.9 7.2 7.1 6.6 6.5 6.4 The linkage analysis of the F2 hybrid males suggests there may be as many as three regions which produce a detectable effect on wing size in Nasonia. It is clear a genetic factor which produces a major effect is tightly linked to the OR123 locus on linkage group IV. There is also support for another region on linkage group I and weaker support for a third on linkage group V. There are two possible explanations for the observed linkage of the N. giraulti wing trait to three linkage groups in the F2 hybrid males. There may be more than one gene with major phenotypic effect involved in the wing size difference or there may be `pseudo-linkage' between the N. giraulti wing trait (Wg) on linkage group IV and a hybrid lethal. Pseudo-linkage The effect of negative epistatic interactions in hybrid backgrounds presents a unique problem in mapping loci in hybrid crosses. Negative epistasis in hybrids often results in lethal gene combinations, affecting the results of linkage analyses because a gene of interest can appear linked to two linkage groups due to the negative epistatic interaction. If a gene of interest is tightly linked to a hybrid lethal, it will also appear to be linked to the region where the epistatic interactor with that hybrid lethal is located. This is illustrated in Fig. 3 where linkage of a trait (i.e. t locus) to a hybrid lethal locus (i.e. lA) creates the appearance of linkage to a mutant marker. If a marker, located on a separate linkage group, is linked to Genetics of species wing size differences the locus (lB) that interacts with lA the marker then will also appear linked to the trait locus. In this model the allele from one species (lA2*) is a recessive lethal when combined with the interactor allele from the other species (lB1*). The `*' indicates a lethal allele. Thus, lA2* lB1* haploid F2 males die. Since the t locus is linked to one interactor, and the marker is linked to the other, pseudo-linkage can occur. Given recombination rates of r1 between lB and m, and r2 between lA and t, the apparent linkage between m and t is rpseudo 2 ÿ r 1 r 2 ÿ 1 ÿ r 1 1 ÿ r 2 : 3 2 As r2 converges on zero (i.e. tight linkage between the trait locus and lA), then rpseudo 1 r 1 3 3 and similarly, as r1 converges on zero (tight linkage between the marker and lB) then rpseudo 1 r2 : 3 4 Thus, the maximal pseudo-linkage expected with a two locus hybrid lethal interaction is rpseudo 1=3: 5 Note also that pseudo-linkage creates an asymmetry in the recovery ratio of the two recombinant genotypes (Fig. 3). Fig. 3 Diagram of pseudo-linkage to a hybrid lethal in a haplodiploid system. (a) The genotype of the F1 hybrid females: t is the loci of interest, lA is the ®rst interactor involved in the hybrid lethal, lB is the second interactor involved in the hybrid lethal; m is a mutant marker. (b) The probability of different genotypes in the F2 males. (c) The apparent frequency of recombination between t and m due to pseudo-linkage. 591 Pseudo-linkage complicates the analysis of phenotypic traits in interspecies crosses. One approach to resolving the involvement of particular genetic regions in a phenotype is to introgress the region into a homogenous genetic background while eliminating or reducing the hybrid deleterious effects through recombination. While N. vitripennis and N. giraulti are capable of hybridization, some hybrid breakdown occurs. Previous studies have demonstrated increased mortality among F2 recombinant hybrids due to negative epistatic interactions between nuclear genes (Breeuwer & Werren, 1995). One of the genes involved in the hybrid lethality (designated la) has been mapped near the OR123 locus (unpublished data). When males carrying the Ng allele (lag*) of this gene also carry the Nv interactor (lbv*) the resulting larvae die. The presence of these hybrid lethals in the Nasonia system complicates the task of mapping the Wg wing trait. Since at least one factor involved in the wing size difference between Nv and Ng is on linkage group IV, near a hybrid lethal, it will show pseudolinkage to the second interactor involved in the nuclear±nuclear lethality. Introgression lines The Ng large wing trait was introgressed into a Nv genetic background by a series of backcrosses. From this line, three sublines homozygous for the N. giraulti wing trait were purebred and characterized (Table 1). The three INTW1 sublines show no signi®cant difference in head size (P > 0.05) or in normalized wing multiple (P > 0.05) among the three sublines. The INTW1 sublines have a mean head width of 9.2 0.6 (n 185), which does not differ signi®cantly from the Nv mean head width (9.2 0.8, n 75) (P > 0.05). This indicates INTW1 males are on average the same size as Nv males. In contrast, INTW1 males have an average normalized wing multiple of 32.7 3.4 (n 185), which is signi®cantly larger than the average normalized wing multiple of Nv males (20.1 2.3, n 75) (P < 0.001). The third species in the genus, N. longicornis, also has wings intermediate to those of N. vitripennis and N. giraulti. Nl has an average normalized wing multiple of 34.3 2.5 (n 25) which does not differ signi®cantly from the average normalized wing multiple of the INTW1 sublines (P > 0.05). Due to the similarities of the IWTW1 sublines, subsequent analysis was performed on one line, IWTW1.1. The wings of INTW1.1 males are 26% wider and 17% longer than Nv males. The Wg trait, introgressed into an Nv background, causes a 63% increase in the average normalized wing multiple when compared to the wildtype Nv strain. This difference accounts for 44% of the difference in the average normalized wing multiples between Nv and Ng (see Fig. 4). The INTW1.1 subline shows a 16% decrease in bristle density relative to Nv males (Table 1) and INTW1.1 bristle densities are intermediate to the Nv and Ng densities (Fig. 2). There J. EVOL. BIOL. 12 (1999) 586±595 ã 1999 BLACKWELL SCIENCE LTD 592 R . F. W E S T O N E T A L . and may be producing lower quantities of a maternal product affecting progeny wing size. Based on the frequency of recombination between OR123 and the Wg trait (Table 2), we estimate the Wg locus to be located on linkage group IV, »1.4 map units from OR123. The results of the linkage analysis indicate that any loci affecting wing size on linkage groups I and V were not introgressed into the INTW1 strain, or that these F2 effects are due to pseudo-linkage. Discussion Darwin (1859) ®rst suggested that selection acts on small differences in phenotypes over time, producing drastically different structures. Darwin's view was contested by Huxley (1860), who claimed that nature can make jumps, thus sparking a debate on the importance of large Fig. 4 The normalized wing multiple of the INTW1 introgression line (subline INTW1.1). The wings of the INTW1 males are clearly intermediate to those of the Nv and Ng[G] parental strains. appears to be no correlation between the bristle density and the normalized wing multiple within a strain but a correlation clearly exists between bristle densities and normalized wing multiples between the Ng, INTW1.1 and Nv strains. Interestingly, while Nl and INTW1.1 males have similar size wings, their bristle densities differ signi®cantly (Nl 67 8, n 25; INTW1.1112 10, n 50) (P < 0.001) indicating that the Wg region is not solely responsible for the intermediate wing phenotype observed in Nl males. Linkage analysis of introgression lines After creation and characterization of the INTW1 sublines (Fig. 4), another linkage analysis was performed to determine which regions responsible for the observed wing size difference in F2 recombinant hybrids had been isolated in the introgression line. The N. giraulti wing trait introgressed into the INTW1 stock shows strong segregation in the cross to OR123, indicating that we have indeed introgressed the N. giraulti wing trait (Wg), responsible for the major difference between the species, into a Nv background (Table 2). The distribution of normalized wing multiples among the F2 hybrids, in the INTW1.1 cross to OR123R, appears bimodal. However, the distribution is shifted inward relative to the parental distributions (P < 0.001 Fig. 5). This shift may be due to the presence of modi®ers of small effect, or the wing phenotype may be in¯uenced by the expression of the maternal genotype (i.e. maternal effects). The F1 hybrids produced in this cross are heterozygous for the Wg trait J. EVOL. BIOL. 12 (1999) 586±595 ã 1999 BLACKWELL SCIENCE LTD Fig. 5 Linkage analysis of the Wg trait in the INTW1.1 line. (a) The normalized wing multiples of the INTW1.1 (n 105) and OR123 (n 100) parental strains; (b) the normalized wing multiples of male forewings found in the F2 males resulting from an INTW1.1 male crossed to an OR123R mutant female (n 120). Genetics of species wing size differences mutations in evolution which has lasted over a century. Fisher (1958) presented the primary argument against the evolutionary importance of mutations with large effect. Fisher claimed large mutations have a very small chance of being favourable because they tend to radically alter the function of a gene or eliminate gene function entirely. Therefore, adaptations must be based on numerous gene substitutions, all of which have relatively small effects. Fisher's theory claims mutations with major phenotypic effects on morphology tend to have deleterious pleiotropic effects, mutations with smaller effects occur more frequently than those with large effects, and that large effect mutations are more likely to be further from the adaptive optimum phenotype. Lande (1983) has developed a quantitative model in support of Fisher's predictions. While mutations of large effect can have negative pleiotropic effects, deleterious pleiotropy may be equally likely for mutations with minor effects (Orr & Coyne, 1992), and the deleterious pleiotropy of mutations with major effects can be ameliorated by the presence of modi®ers (Maynard Smith, 1983). Furthermore, Fisher's model assumes the population is already near the selective optimum. Otherwise, mutations with large effects are not necessarily farther from the optimum than mutations with small effects. In addition, Kimura (1983) argued that while mutations of large effect are less likely to be favourable than mutations of small effect, mutations with major effects (that move the phenotype close to the optimum) are ®xed more easily. An interesting pattern has been observed during the evolution of insecticide resistance in natural populations of the blow¯y, Lucilia cuprena (McKenzie et al., 1990; Clarke, 1997). Initially, selection favoured the increase of a gene of major effect imparting resistance to organophosphorous insecticides. However, the resistant allele had negative (pleiotropic) side-effects. Selection subsequently favoured the increase of a modi®er that ameliorated these negative pleiotropic effects. Turning the clock forward, one might expect the accumulation of additional modi®ers that ameliorate other and/or weaker negative side-effects, as well as modi®ers that re®ne the insecticide resistance trait. Thus, the genetic architecture of this trait may be expected to change over time, from relatively simple with genes of major effect to more complex (e.g. major gene effects plus many modi®ers). We expect that this pattern will be common in nature, particularly when the original phenotype was subject to strong selection. In this study we have shown a signi®cant proportion of a morphological difference between two species of Nasonia is due to a single gene or a small region of a few tightly linked genes. Two conclusions can clearly be drawn from this study. First, the Ng wing trait is not highly polygenic since it only shows strong linkage to two linkage groups (I and IV) in F2 hybrid analyses. Second, there is clearly a major factor located on linkage 593 group IV. Crosses between Nv and Ng result in F2 recombinant hybrid males which show a non-normal, nonunimodal distribution in forewing size (Fig. 3). The apparent bimodality of this distribution indicates the wing size difference between Nv and Ng is primarily due to a single or few mutations of large phenotypic effect. It is still not clear how many genes are involved in the Ng wing trait; however, it is clear that there are major factors involved. After determining that a genetic region of large effect is involved in the species wing size difference, we selected for large wings while introgressing Ng genes into a Nv genetic background (creating the INTW1 strain). Analysis of the INTW1 strain indicates that it carries the Wg large wing region linked to OR123. Linkage analysis shows that the INTW1 large wing trait is caused by a gene of large effect (or a region of tightly linked genes) which is located on linkage group IV, »2.1 map units from the OR123 mutant marker. This region of the Ng genome is responsible for »44% of the difference in average normalized wing multiple between the Nv and Ng[V] parental strains. We refer to this gene or region of genes as Wg. Wg actually causes a 63% increase in the average normalized wing multiple when compared to the Nv strain into which it was introgressed. Thus, we have introgressed one of the major genes involved in Ng large wings into an Nv nuclear background. The results of the F2 hybrid analysis indicate that there is another locus affecting wing size on linkage group I (Fig. 5), which was not introgressed into INTW1. Since the N. vitripennis wing trait shows strong segregation with RED833 this effect is not likely to be caused by pseudolinkage. If there is another locus with a major effect on wing size located on linkage group I, nearly all of the variation in wing size between Nv and Ng may be controlled by these two regions. It has previously been shown, in Drosophila, that bristle number in wings is correlated with cell number in wings (Dobzhansky, 1929). Nv has a bristle density approximately three times that of Ng. Thus, if the trend observed in Drosophila proves true in Nasonia, then it appears the wing cells in Ng are larger than in Nv. Additionally the introgression line, INTW1, has an intermediate bristle density to the parental lines (Fig. 4). Wg appears to primarily affect wing width. Wings show a 26% increase in normalized wing width in the INTW1 stock, but only a 17% increase in normalized wing length (Fig. 5). This may be due to increased cell width or an increase in the number of cells on the width axis of the wing. The bristle density data seem to support wider cells, but further studies will have to be conducted to determine the relationship between bristle number and cell number in Nasonia wings. Previous research shows that ploidy effects cell size in a wide range of species (Cavalier-Smith, 1985). The ploidy of Nv and Ng wings, and the possibility of alternative mechanisms for regulating cell size, remain to be investigated. J. EVOL. BIOL. 12 (1999) 586±595 ã 1999 BLACKWELL SCIENCE LTD 594 R . F. W E S T O N E T A L . We have created an introgression line (INTW1) which has wings intermediate in size to those of N. vitripennis and N. giraulti. N. longicornis also has a wing size intermediate between Nl and Ng males. The average normalized wing multiple of Nl males does not differ signi®cantly from that of INTW1 males, which raises a question: is the Wg locus responsible for the difference in wing size observed between N. vitripennis and N. longicornis? Nasonia belongs to the Dibrachys group in the Pteromalidae, subfamily Pteromalinae. This includes wasps in the genera Dibrachys, Dibrachoides and Muscidifurax. Among these genera, vestigial-winged males have only been found in Nasonia (Wallace, 1973). It therefore seems the ancestral state of the wing is the large form, and N. vitripennis has evolved smaller wings either due to mutational degradation or due to selection for smaller wings. N. giraulti and N. longicornis are sister species and N. vitripennis is more divergent. However, all are `young' species, having diverged in the Pleistocene (0.2±1 Ma) (Campbell et al., 1993). The adaptive signi®cance of male wing size in Nasonia remains unclear. The evolution of small wing size in males could be either an adaptive trait or the result of mutational degradation of an unused structure. Nevertheless, the loss of long-range male dispersal associated with wing size reduction is consistent with the mating population structure of these wasps. All three species of Nasonia have highly subdivided populations in nature (Werren, 1983; Darling & Werren, 1990) and show strongly female-biased sex ratios (Skinner, 1983; Werren, 1983; Drapeau & Werren, 1999), indicative of local mating (Hamilton, 1967). If mating opportunities for males are con®ned to the local area where they emerge, then selection for nondispersing males is expected. Selection for nondispersing males could result in males with small vestigial wings. However, this argument would seem to apply equally to all three species, whereas small wings evolved in Nv and Nl, but not in Ng. Preliminary data on male dispersal in Nasonia have revealed a difference in ¯ight abilities between the species. Ng males ¯y, but have low ¯ight tendency. Males of different strains of Nl show variable ¯ight tendency. Nv males do not ¯y, nor do they exhibit ¯ight behaviours (i.e. raising wings) (unpublished data). These results imply that reduction of ¯ight behaviour precedes the loss of wings. Evolution of small wings in Nv could have been selectively favoured due to advantages in male±male competition. Consistent with this view, Nv males are more aggressive than are Nl or Ng males, and Nv shows less female-biased sex ratios, indicative of more mate competition. In contrast, Ng males and females typically mate within the host, where inbreeding is likely to be high and mate competition low (Drapeau & Werren, 1997). Interestingly, Nv males use their vestigial wings in aggressive displays by raising them up off their backs, a behaviour not observed in Ng. These species differ in a J. EVOL. BIOL. 12 (1999) 586±595 ã 1999 BLACKWELL SCIENCE LTD number of interesting respects, including courtship and mating, sex ratio, male aggression, ¯ight behaviour, morphology (wing size, antennal shape) and host preference. An integrative approach combining behavioural, genetic and molecular genetic studies promises to be useful in revealing how these differences have evolved in the species complex. The ability to move genetic regions between the species will be particularly useful for such studies. It will be interesting to determine how often phenotypic differences between these species are due to genes of large effect vs. the accumulation of many genes of small effect. Acknowledgments Thanks are extended to Charlotte Lee and Dan Williams for their contribution to this research, and to C. Arboleda and B. McAllister for general assistance. Thanks to C. Brett, S. Bordenstein, V. Calhoun, M. Drapeau, C. Jones, A. Orr, D. Presgraves and M. Perfeccti, who provided helpful comments on the manuscript, and R. Hannigan helped with statistical and graphical programs. C. Jones and A. Orr kindly provided their derivation for the minimum number of genes estimate for haplodiploidy. References Bradshaw, H.D., Wilbert, S.M., Jr, Otto, K.G. & Schemske, D.W. 1995. Genetic mapping of ¯oral traits associated with reproductive isolation in monkey¯owers (Mimulus). Nature 376: 762±765. Breeuwer, J.A. & Werren, J.H. 1990. Microorganisms associated with chromosome destruction and reproductive destruction and reproductive isolation between two insect species. Nature 346: 558±560. Breeuwer, J.A. & Werren, J.H. 1995. Hybrid breakdown between two haplodiploid species: the role of nuclear and cytoplasmic genes. Evolution 49: 705±717. Campbell, B.C., Steff, J.D., n-Campell, -e. & Werren, J.H. 1993. Phylogeny of the Nasonia species complex (Hymenoptera: Pteromalidae) inferred from an internal transcribed spacer (ITS2) and 28S rDNA sequences. Ins. Mol. Biol. 2: 225±237. Cavalier-Smith, T. 1985. Cell volume and the evolution of eukayotic genome size. In: The Evolution of Genome Size (ed. T. Calvalier-Smith), pp. 105±184. John Wiley, New York. Clarke, G.M. 1997. The genetics and molecular basis of developmental stability: The Lucilia story. Trends Ecol. Evolution 12: 89±91. Clarke, C.A. & Sheppard, P.M. 1960. The evolution of mimicry in the butter¯y Papilio dardanus. Heredity 14: 163±173. Coyne, J.A., Rux, J. & David, J.R. 1991. Genetics of morphological differences and hybrid sterility between Drosophila sechellia and its relatives. Genet. Res. 57: 113±122. Crow, J.F. 1957. Genetics of insect resistance to chemicals. Ann. Rev. Ent. 2: 227±246. Darling, D.C. & Werren, J.H. 1990. Biosystematics of Nasonia (Hymenoptera: Pteromalidae): two new species reared from bird's nests in North America. Ann. Ent. Soc. Am. 83: 352±370. Darwin, C. 1859. On the Origin of Species. John Murray, London. Genetics of species wing size differences Dobzhansky, T. 1929. The in¯uence of the quantity and quality of chromosomal material on the size of the cells in Drosophila melanogaster. Arch. Entwicklungsmech. Org 115: 363±379. Drapeau, M.D. & Werren, J.H. 1998. Differences in population structure and sex ratio between three sibling species of Nasonia. Evol. Ecol. Res. in press. Fisher, R.A. 1958. The Genetical Theory of Natural Selection, 2nd edn. Sinauer, Sunderland, Mass. Gottlieb, L.D. 1984. Genetics and morphological evolution in plants. Am. Nat. 123: 681±709. Hamilton, W.D. 1967. Extraordinary sex ratios. Science 156: 477± 478. Hat®eld, T. 1997. Genetic divergence in adaptive characters between sympatric species of stickleback. American Naturalist 149: 1009±1029. Huxley, T.H. 1860. The origin of species. Westminster Review 17: 541±570. Kimura, M. 1983. The Neutral Theory of Molecular Evolution. Cambridge University Press, Cambridge. Lande, R. 1981. The minimum numbers of genes contributing to quantitative variation between and within populations. Genetics 99: 541±553. Lande, R. 1983. The response to selection on major and minor mutations affecting a metrical trait. Heredity 50: 47±65. Lees, D.R. 1981. Industrial melanism: genetic adaptation of animals to air pollution. In: Genetic Consequences of Man Made Change (J. A. Bishop & L. M. Cook eds), pp. 129±176. Academic Press, London. Liu, J., Merger, J.M., Stam, L.F., Gibson, G.C., Zeng, Z.B. & Laurie, C.C. 1996. Genetic analysis of a morphological shape difference in male genitalia of Drosophila simulans and Drosophila mauritiana. Genetics 142: 1129±1145. Maynard Smith, J. 1983. The genetics of stasis and punctuation. Annu. Rev. Genet. 17: 11±25. McKenzie, J.A., Batterham, P. & Baker, M.L. 1990. Fitness and asymmetry modi®cation as an evolutionary process. A study in the Australian Sheep Blow¯y, Lucilia cuprena and Drosophila 595 melanogaster. In: Ecological and Evolutionary Genetics of Drosphila (J. S. S. Barker, W. T. Starmer & R.J. MacIntyre, eds), pp. 57± 73. Plenum Press. McNair, M. 1981. Tolerance of higher plants to toxic materials. In: Genetic Consequences of Man Made Change (J. A. Bishop & L. M. Cook, eds), pp. 177±208. Academic Press, London. Orr, H.A. & Coyne, J.A. 1992. The genetics of adaptation: a reassessment. American Naturalist 140: 725±742. Piper, L.R. & Shrimpton, A.E. 1989. The quantitative effects of genes which in¯uence metric traits. In: Evolution and Animal Breeding (W. G. Hill & T. F. C. Mackay, eds), pp. 147±151. C. A. B. International, Wallingford, UK. Sheppard, P.M. 1962. Some aspects of the geography, genetics, and taxonomy of a butter¯y. Tax. Geog. 4: 135±152. Shrimpton, A.E. & Robertson, A. 1988. The isolation of polygenic factors controlling bristle score in Drosophila melanogaster. II. Distribution of third chromosome bristle effects within chromosome sections. Genetics 11: 49±78. Skinner, S.W. 1983. Extra chromosomal sex ratio factors in the parasitoid was Nasonia ( Mormoniella) Vitripennis. PhD thesis, University of Utah, Salt Lake City, Utah. Spurway, H. 1953. Genetics of speci®c and subspeci®c differences in European newts. Symp. Soc. Experimental Biol. 7: 200± 237. Turner, J.R.G. 1981. Adaptation and evolution in Heliconius: a defense of NeoDarwinism. Ann. Rev. Ecol. Syst 12: 99±121. Wallace, G.E. 1973. New Pteromalidae of the Dibrachys group (Hymenoptera: Chalcidoidea) with a key to genera. Ann. Carnegie Mus. 44: 171±181. Werren, J.H. 1983. Sex ratio evolution under local mate competition in a parasitic wasp. Evolution 37: 116±124. Whiting, A.R. 1967. The biology of the parasitic wasp Mormoniella vitripennis [ Nasonia brevicornis] (Walker). Quart. Rev. Biol. 42: 333±406. Received 20 July 1998; accepted 23 July 1998 J. EVOL. BIOL. 12 (1999) 586±595 ã 1999 BLACKWELL SCIENCE LTD