* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download File
Survey
Document related concepts
Transcript
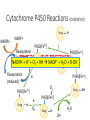
Detoxification by the Liver Phase I and II reactions Xenobiotics • Foreign chemical substance • Can be absorbed across lungs, skin or ingested • Drugs are considered xenobiotics • Excreted in bile, urine, sweat, breath Pharmacologically Active Compounds • Lipophilic • To pass through plasma membranes to reach metabolising enzymes • Non-ionised at pH7.4 • Bound to plasma proteins • To be transported in the blood Enzymes • Microsomal and Non-microsomal enzymes • BOTH be involved in phase I and II reactions • Microsomal enzymes mainly Phase I • Non-microsomal mainly Phase II Microsomal enzymes • Located on smooth endoplasmic reticulum • Phase I reactions – biotransform substances • Phase II – glucuronidation • Activity can be induced or inhibited • Drugs, food, age, bacteria, alcohol • Examples: Cytochrome P450 (CYPs), Flavin monooxygenase (FMOs), UDPglucuronosyltransferase (UGT) Non-microsomal enzymes • Located in cytoplasm and mitochondria • Non-specific so Phase I and Phase II reactions • All conjugation reactions EXCEPT GLUCURONIDATION • Non-inducible • Genetic polymorphic – affects metabolism • Examples: protein oxidases, esterases, amidases, conjugases (transferases), alcohol dehydrogenase, aldehyde dehydrogenase Drug Metabolism • Why? • Most drugs excreted by kidneys • Lipophilic drugs not effectively removed • AIM: To make drugs more polar • Mostly occur in liver • 2 mechanisms – phase I and II reactions • Usually sequentially Phase I • Non-synthetic catabolic reactions • Oxidation, Reduction, Hydrolysis Phase I reactions • Oxidation • • • • Hydroxylation (add –OH) Dealkylation (remove –CH side chains) Deamination (remove –NH) Hydrogen removal • Reduction • Add hydrogen (saturate unsaturated bonds) • Hydrolysis • Split amide and ester bonds Phase I • Non-synthetic catabolic reactions • Oxidation, Reduction, Hydrolysis • Known as ‘functionalisation’ • • • • Introduces reactive group to drug Includes adding or exposing –OH, -SH, -NH2, -COOH Product usually more reactive Small increase in hydrophilicity • Mainly occur in the liver • Mainly catalysed by Cytochrome P450 • Drug has to get into cell – more lipophilic Cytochrome P450 Enzymes • Type of microsomal enzyme • Phase I reaction • Haem group to oxidise substances • Products more water soluble Cytochrome P450 • Large family with prefix CYP – known as isoforms/ isozymes • 1st number – indicates the family the enzyme belongs to • Letter – to indicate subfamilies • 2nd number – individual genes involved • Isoforms catalyse different reactions (specificity) • Some important isozymes – CYP1A2, CYP2C9, CYP2C19, CYP2D6,CYP2E1, CYP3A4 Cytochrome P450 Reductase • Flavoprotein • Contains both Flavin adenine dinucleotide (FAD) and Flavin mononucleotide (FMN) • FAD – accepts electrons from NADPH • FMN – electron donor to CYPs Cytochrome P450 Reactions (oxidation) Drug NADPH H NADP+ P450[Fe3+] Flavoprotein (oxidised) TIME TODrug PUTHIT NADPH + H+ Flavoprotein (reduced) + O2 TOGETHER! + RH NADP+ + H O + R-OH 2 Drug OH e- P450[Fe3+] O2 P450[Fe2+] Drug P450[Fe3+] H eO2 Drug P450[Fe2+] Drug H H2O 2H+ OH Remember! • Non-microsomal enzymes • • • • Alcohol dehydrogenase Aldehyde dehydrogenase Reduction Hydrolysis • Phase I reactions can: • • • • Inactivate drug Further activate drug Activate drug from pro-drug (inactive form) Make a drug into a reactive intermediate (could be carcinogenic or toxic) Phase II • Synthetic anabolic reactions • Glucuronidation, sulfation, Glutathione conjugation, amino acid conjugation, acetylation, methylation, water conjugation • Known as ‘conjugation’ reactions • Attachment of substituent groups (endogenous molecules) • Usually inactivate products • Catalysed by transferases • Significantly increase hydrophilicity for renal excretion • Also mainly in the liver Glucuronidation • Glucuronosyltransferase (UGT) – microsomal enzyme, phase II reaction. • Uridine diphospho-glucuronic acid (UDPGA) needed to conjugate glucuronic acid. • Pathway for bilirubin conjugation and drugs including corticosteroids & paracetamol. Glucuronidation Reaction UDPGA Drug UGT Uridine diphosphate Drug Glucuronide MORE HYDROPHILIC! Remember! • Most phase II reactions involved non-microsomal enzymes • Mostly found in the cytoplasm or mitochondria Elimination (usually polar drug, excreted unchanged) DRUG Phase II Elimination (functionalised without Phase I) Phase I Phase II Elimination Aspirin • Analgesic • NSAID • Non steroidal anti-inflammatory drug • Antiplatelet • Irreversibly inhibits cyclooxygenase (COX) Aspirin Phase 1 metabolism • Prodrug so it is activated upon metabolization • Hydrolysis reaction • Aspirin (+H20) → Salcylic acid + Ethanoic acid • Salcyclic acid is the active anti-inflammatory and analgesic Aspirin Phase 2 metabolism • Conjugated with glycine or glucuronic acid • Forms a range of ionised metabolites • Excreted in the urine Paracetamol • Also known as Acetaminophen • Analgesic • Antipyretic agent Paracetamol Metabolism • Predominantly PHASE 2 metabolism • Conjugation with glucuronic acid and sulphate Paracetamol toxicity • If stores of glucuronic acid and sulphate are running low… • Undergoes PHASE 1 metabolism (oxidation) to produce toxic NAPQI • This is removed by conjugation with glutathione • In overdose stores of glutathione can run low leading to toxicity • Treated with N-Acetyl Cysteine Alcohol metabolism • Ethanol → Acetaldehyde → Acetate (ADH) (ALDH) • Acetate CO2 + H2O • ADH – Alcohol Dehydrogenase • ALDH – Aldehyde Dehydrogenase • Operate at different speeds in different people • Acetaldehyde • Carcinogenic • High levels: Facial flushing, rapid heartbeat, nausea Any Questions?






























![[4-20-14]](http://s1.studyres.com/store/data/003097962_1-ebde125da461f4ec8842add52a5c4386-150x150.png)







