Atomic and Molecular Physics for Physicists Ben-Gurion University of the Negev
... • Quantum mechanics was born out of several experimental problems such as the UV catastrophe, and the quantized absorption and emission spectrum of the atoms (light and Frank-Hertz) • First good predictions were achieved when one assumed quantization of the atomic oscillations in the bulk (Plank), a ...
... • Quantum mechanics was born out of several experimental problems such as the UV catastrophe, and the quantized absorption and emission spectrum of the atoms (light and Frank-Hertz) • First good predictions were achieved when one assumed quantization of the atomic oscillations in the bulk (Plank), a ...
Modern Physics Lesson 3
... This can obviously only be used for objects with mass (i.e., NOT photons). It is usually used for electrons and protons, since they are the only objects that display noticeable wave-like properties. Normal sized objects (like a book or a basketball) also have a deBroglie wavelength, but it is so sma ...
... This can obviously only be used for objects with mass (i.e., NOT photons). It is usually used for electrons and protons, since they are the only objects that display noticeable wave-like properties. Normal sized objects (like a book or a basketball) also have a deBroglie wavelength, but it is so sma ...
Chemistry 1 Concept 5 “Electrons in Atoms” Study Guide
... 1s22s22p63s23p4 _______________ 1s22s22p63s23p64s23d104p3 ____________ 2. Specific wavelengths of light seen through a prism that are made when high voltage current is passed through a tube of hydrogen gas at low pressure is _________________ 3. The energy of a photon is related to its _____________ ...
... 1s22s22p63s23p4 _______________ 1s22s22p63s23p64s23d104p3 ____________ 2. Specific wavelengths of light seen through a prism that are made when high voltage current is passed through a tube of hydrogen gas at low pressure is _________________ 3. The energy of a photon is related to its _____________ ...
Synopsis
... Don’t try to work out the momentum of a high speed particle and hence its de-Broglie wavelength using its rest mass. Mas increase significantly as the speed of the particle approaches the speed of light! ...
... Don’t try to work out the momentum of a high speed particle and hence its de-Broglie wavelength using its rest mass. Mas increase significantly as the speed of the particle approaches the speed of light! ...
File - SPHS Devil Physics
... 1. Essential Idea: The microscopic quantum world offers a range of phenomena, the interpretation and explanation of which require new ideas and concepts not found in the classical world. 2. Nature Of Science: a. Observations: Much of the work towards a quantum theory of atoms was guided by the need ...
... 1. Essential Idea: The microscopic quantum world offers a range of phenomena, the interpretation and explanation of which require new ideas and concepts not found in the classical world. 2. Nature Of Science: a. Observations: Much of the work towards a quantum theory of atoms was guided by the need ...
Quantum Mechanics and the Bohr Model - slater science
... waves superimposed on each other. Both waves should have the same amplitude but different frequencies. • Draw another horizontal line and two waves with the same wavelength but different amplitudes. ...
... waves superimposed on each other. Both waves should have the same amplitude but different frequencies. • Draw another horizontal line and two waves with the same wavelength but different amplitudes. ...
File
... b. atomic absorption spectra c. atomic emission spectra d. the deflection of cathode rays by an electric field e. absorption of beta particles 3. Rutherford's gold foil experiment showed that the atom is mostly empty space because a. some alpha particles were reflected right back b. some alpha parti ...
... b. atomic absorption spectra c. atomic emission spectra d. the deflection of cathode rays by an electric field e. absorption of beta particles 3. Rutherford's gold foil experiment showed that the atom is mostly empty space because a. some alpha particles were reflected right back b. some alpha parti ...
Quantum Notes
... position and velocity/momentum of an electron at any time. • Because whatever you use to view it (light or radiation) would change the position or momentum ...
... position and velocity/momentum of an electron at any time. • Because whatever you use to view it (light or radiation) would change the position or momentum ...
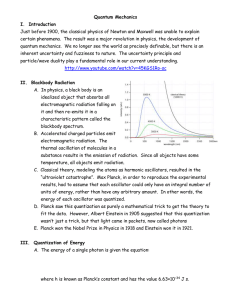
Quantum Mechanics I. Introduction Just before 1900, the classical
... II. Blackbody Radiation A. In physics, a black body is an idealized object that absorbs all electromagnetic radiation falling on it and then re-emits it in a characteristic pattern called the blackbody spectrum. B. Accelerated charged particles emit electromagnetic radiation. The thermal oscillation ...
... II. Blackbody Radiation A. In physics, a black body is an idealized object that absorbs all electromagnetic radiation falling on it and then re-emits it in a characteristic pattern called the blackbody spectrum. B. Accelerated charged particles emit electromagnetic radiation. The thermal oscillation ...
Lec-22_Strachan
... At long wavelengths, the match is good At short wavelengths, classical theory predicted infinite energy At short wavelengths, experiment showed no energy This contradiction is called the ultraviolet catastrophe ...
... At long wavelengths, the match is good At short wavelengths, classical theory predicted infinite energy At short wavelengths, experiment showed no energy This contradiction is called the ultraviolet catastrophe ...
Quantum Mechanics Lecture 1 Dr. Mauro Ferreira
... • Consider the following experiment: “classical” particles are allowed through a narrow gap. The blue curve displays how they are spatially distributed ... and now through two separate gaps. The distribution is just a simple addition of the two individual distributions ...
... • Consider the following experiment: “classical” particles are allowed through a narrow gap. The blue curve displays how they are spatially distributed ... and now through two separate gaps. The distribution is just a simple addition of the two individual distributions ...
Lecture 24: Quantum mechanics
... o Electron orbit radius is also quantized (r—n2) o Electron energy is also quantized consistent with Planck theory ...
... o Electron orbit radius is also quantized (r—n2) o Electron energy is also quantized consistent with Planck theory ...
Chapter 5 PPT/Notes A
... • Wavelength is the length of a wave from one location to the same location in the next wave…crest to crest for example. • Amplitude is the vertical distance from origin to crest or origin to trough. • The trough is the ‘bottom-point’ of a wave and the crest is the ‘peak’ of a wave. • Frequency is h ...
... • Wavelength is the length of a wave from one location to the same location in the next wave…crest to crest for example. • Amplitude is the vertical distance from origin to crest or origin to trough. • The trough is the ‘bottom-point’ of a wave and the crest is the ‘peak’ of a wave. • Frequency is h ...
Slide 1 - s3.amazonaws.com
... 7.4 Quantum Mechanics Physicists were both mystified and intrigued by Bohr’s theory. They questioned why the energies of hydrogen electron are quantized, or, why is the electron in a Bohr atom restricted or orbiting the nucleus at certain fixed distance? For a decade there is no logical explanation ...
... 7.4 Quantum Mechanics Physicists were both mystified and intrigued by Bohr’s theory. They questioned why the energies of hydrogen electron are quantized, or, why is the electron in a Bohr atom restricted or orbiting the nucleus at certain fixed distance? For a decade there is no logical explanation ...
dual nature of light
... Diffraction :➢Light bends around the corner but Newton refused this phenomenon. ...
... Diffraction :➢Light bends around the corner but Newton refused this phenomenon. ...
Quantum Theory of Light. Matter Waves.
... aspects of the reality. However, the physical reality arises from small-scale world of atoms and molecules, electrons and nuclei. Electrons behave as particles because they have charge and mass, but moving electrons also show evidence of behaving as waves (diffraction, interference). The wave-partic ...
... aspects of the reality. However, the physical reality arises from small-scale world of atoms and molecules, electrons and nuclei. Electrons behave as particles because they have charge and mass, but moving electrons also show evidence of behaving as waves (diffraction, interference). The wave-partic ...
Development of Quantum Mechanics Waves
... If light is a wave with particle properties, are electrons particles with wave properties? C. J. Davisson and L. H. Germier at Bell labs proved that electrons produce diffraction patterns and verified deBroglies hypothesis ...
... If light is a wave with particle properties, are electrons particles with wave properties? C. J. Davisson and L. H. Germier at Bell labs proved that electrons produce diffraction patterns and verified deBroglies hypothesis ...
Chemistry Science Notebook
... The quantum concept concludes that matter can gain or lose only in small, specific amounts called ...
... The quantum concept concludes that matter can gain or lose only in small, specific amounts called ...
Modern physics 2330
... 5- (..) A black hole is an object of sufficiently high density can trap light forever. 6- ( ) Millikan’s oil drop experiment is inaccurate because it is difficult to measure the speed of drop. 7- (..) Thompson cloud chamber experiment is inaccurate because the theory applies only to a single particl ...
... 5- (..) A black hole is an object of sufficiently high density can trap light forever. 6- ( ) Millikan’s oil drop experiment is inaccurate because it is difficult to measure the speed of drop. 7- (..) Thompson cloud chamber experiment is inaccurate because the theory applies only to a single particl ...