Answers to Critical Thinking Questions 4
... a) 1s22s22p63s23p44s1 – the 3p orbitals were not completely filled before electrons were added to 4s (violating the Aufbau principle). The correct configuration is 1s22s22p63s23p5 b) 1s22s22p63s23p7 – the maximum number of electrons in 3p is 6 (violating the Pauli exclusion principle). The correct c ...
... a) 1s22s22p63s23p44s1 – the 3p orbitals were not completely filled before electrons were added to 4s (violating the Aufbau principle). The correct configuration is 1s22s22p63s23p5 b) 1s22s22p63s23p7 – the maximum number of electrons in 3p is 6 (violating the Pauli exclusion principle). The correct c ...
Graphene
... directional and energy dependence of transmission in graphene. • The problem #1 is manufacturing of clean samples. • Most of the physics observed so far is a single particle one. • Many-body effects are observed in FQHE in strong magnetic fields. The role of bending fluctuations is not very clear, t ...
... directional and energy dependence of transmission in graphene. • The problem #1 is manufacturing of clean samples. • Most of the physics observed so far is a single particle one. • Many-body effects are observed in FQHE in strong magnetic fields. The role of bending fluctuations is not very clear, t ...
Arrangement of Electrons in Atoms (Chapter 4) Notes
... B. In 1926, the Austrian physicist Erwin Schrodinger used the hypothesis that electrons have a dual wave/particle nature to develop an equation that treated electrons in atoms as waves. Schrodinger’s equation results in a series of so called wave functions, represented by the letter (psi). Althou ...
... B. In 1926, the Austrian physicist Erwin Schrodinger used the hypothesis that electrons have a dual wave/particle nature to develop an equation that treated electrons in atoms as waves. Schrodinger’s equation results in a series of so called wave functions, represented by the letter (psi). Althou ...
Chapter 3 Atomic Structure
... correlate to the distance that an electron is from an atom’s nucleus. Sublevels (subshells): Each principle energy level (n) is divided into n sublevels. Orbitals: Orbitals are a region in space representing a high probability of locating an electron. Each sublevel has one or more orbital. ...
... correlate to the distance that an electron is from an atom’s nucleus. Sublevels (subshells): Each principle energy level (n) is divided into n sublevels. Orbitals: Orbitals are a region in space representing a high probability of locating an electron. Each sublevel has one or more orbital. ...
PHYS13071 Assessment 2012
... 6. Quantum Computing Although the foundation of quantum theory was developed in the 1920s, the last decade has seen years of great advances in quantum technology, especially in nanotechnology. According to the “Moore’s Law”, the number of transistors on computer chips, and hence their computational ...
... 6. Quantum Computing Although the foundation of quantum theory was developed in the 1920s, the last decade has seen years of great advances in quantum technology, especially in nanotechnology. According to the “Moore’s Law”, the number of transistors on computer chips, and hence their computational ...
Direct Coulomb and Exchange Interaction in Artificial Atoms
... l 苷 0, 61, 62, . . . is the quantum number for angular momentum. h̄v0 is the lateral confining energy and h̄vc 苷 eB兾mⴱ is the cyclotron energy. Each FD state is spin degenerate. At B 苷 0 T the FD spectrum has sets of states with increasing degeneracy [see Fig. 2(c)]. This degeneracy is lifted on inc ...
... l 苷 0, 61, 62, . . . is the quantum number for angular momentum. h̄v0 is the lateral confining energy and h̄vc 苷 eB兾mⴱ is the cyclotron energy. Each FD state is spin degenerate. At B 苷 0 T the FD spectrum has sets of states with increasing degeneracy [see Fig. 2(c)]. This degeneracy is lifted on inc ...
Hybrid_Quantu_Classic_Dynamics!!
... • Hydride transfer from NADPH cofactor to DHF substrate • Calculated KIE (kH/kD) is consistent with experimental value of 3 • Calculated rate decrease for G121V mutant consistent with experimental value of 160 • = 0.88 (dynamical recrossings occur but not significant) ...
... • Hydride transfer from NADPH cofactor to DHF substrate • Calculated KIE (kH/kD) is consistent with experimental value of 3 • Calculated rate decrease for G121V mutant consistent with experimental value of 160 • = 0.88 (dynamical recrossings occur but not significant) ...
Word - UNSW Newsroom
... AM: We are already working on the next logical step, which is to couple two qubits together to demonstrate a quantum logic operation. The way this is obtained is, again, peculiar of a truly quantum system. We will put two atoms so close together that the electrons bound to them start to “overlap”, s ...
... AM: We are already working on the next logical step, which is to couple two qubits together to demonstrate a quantum logic operation. The way this is obtained is, again, peculiar of a truly quantum system. We will put two atoms so close together that the electrons bound to them start to “overlap”, s ...
An Ontological Interpretation of the Wave Function - Philsci
... where position (x2 , y2 , z2 ) is the same as position (x2 , y2 , z2 ), physical entities 1 and 2 are no longer entangled, while physical entity 1 with mass m1 and charge Q1 still jumps discontinuously between positions (x1 , y1 , z1 ) ...
... where position (x2 , y2 , z2 ) is the same as position (x2 , y2 , z2 ), physical entities 1 and 2 are no longer entangled, while physical entity 1 with mass m1 and charge Q1 still jumps discontinuously between positions (x1 , y1 , z1 ) ...
Decoherence in Excited Atoms by Low-Energy Scattering
... Current experimental techniques have allowed the manipulation of atomic systems to previously unthinkable degrees, paving the way to the development of new technologies and the observation of very small quantum effects. One such technology is the quantum computer; trapped-ion systems have been imple ...
... Current experimental techniques have allowed the manipulation of atomic systems to previously unthinkable degrees, paving the way to the development of new technologies and the observation of very small quantum effects. One such technology is the quantum computer; trapped-ion systems have been imple ...
AP Atomics Class Packet Unit 2 - Ms. Drury`s Flipped Chemistry
... will be deflected more from the straight-line path. By comparing the amount or degree of deflection for the particles in a sample of a given element to the standard deflection angles for a sample of carbon-12, atomic masses* can be determined. *These are relative atomic masses – relative to the stan ...
... will be deflected more from the straight-line path. By comparing the amount or degree of deflection for the particles in a sample of a given element to the standard deflection angles for a sample of carbon-12, atomic masses* can be determined. *These are relative atomic masses – relative to the stan ...
Quantum Field Theory - damtp
... There is no mechanism in standard non-relativistic quantum mechanics to deal with changes in the particle number. Indeed, any attempt to naively construct a relativistic version of the one-particle Schrödinger equation meets with serious problems. (Negative probabilities, infinite towers of negativ ...
... There is no mechanism in standard non-relativistic quantum mechanics to deal with changes in the particle number. Indeed, any attempt to naively construct a relativistic version of the one-particle Schrödinger equation meets with serious problems. (Negative probabilities, infinite towers of negativ ...
Unit 4 Notes
... F. Why does it matter that an electron behaves as both particle and wave? 1) The fact that electrons behave as waves leads to some odd observations, like: 2) Heisenberg’s uncertainty principle- it is impossible to know exactly both the of a particle at the same time. a. This limitation is critical i ...
... F. Why does it matter that an electron behaves as both particle and wave? 1) The fact that electrons behave as waves leads to some odd observations, like: 2) Heisenberg’s uncertainty principle- it is impossible to know exactly both the of a particle at the same time. a. This limitation is critical i ...
Determining g-factors in Rubidium-85 and Rubidium
... which converts the intensity of light to a voltage. B= ...
... which converts the intensity of light to a voltage. B= ...
Lecture 2 Hamiltonian operators for molecules CHEM6085: Density
... Please note that in all of the questions below and for the rest of the course, whenever we mention “electronic molecular Hamiltonian” we assume the molecular Hamiltonian operator after the application of the BO approximation ...
... Please note that in all of the questions below and for the rest of the course, whenever we mention “electronic molecular Hamiltonian” we assume the molecular Hamiltonian operator after the application of the BO approximation ...
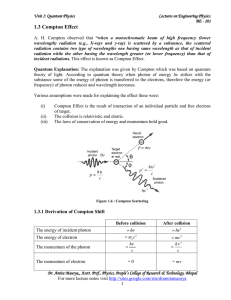
Physics - University of Calcutta
... de Broglie hypothesis. Electron double-slit experiment. Compton effect, Davisson-Germer experiment, Heisenberg’s uncertainty principle (statement) with illustrations. Concept of wave function as describing the dynamical state of a single particle. Group and phase velocities, classical velocity of a ...
... de Broglie hypothesis. Electron double-slit experiment. Compton effect, Davisson-Germer experiment, Heisenberg’s uncertainty principle (statement) with illustrations. Concept of wave function as describing the dynamical state of a single particle. Group and phase velocities, classical velocity of a ...