![[2011 question paper]](http://s1.studyres.com/store/data/008881811_1-8ef23f7493d56bc511a2c01dcc81fc96-300x300.png)
Electron configuration Jeopardy
... 400 – what is the relationship between frequency, wavelength, and energy? The higher the frequency, the shorter the wavelength, the higher the energy 500 – What are the 4 characteristics of a wave? Amplitude, frequency, wavelength, and speed of light Quantum Theory 100 – What are quanta? A small bit ...
... 400 – what is the relationship between frequency, wavelength, and energy? The higher the frequency, the shorter the wavelength, the higher the energy 500 – What are the 4 characteristics of a wave? Amplitude, frequency, wavelength, and speed of light Quantum Theory 100 – What are quanta? A small bit ...
Chp.23 Outline - Redlands High School
... Chapter 23 Atomic Physics Lecture Questions: 1) What is a photon? Do all photons have the same energy? What is a quantum value? Give some examples of other quantum values besides the photon. Write the equation relating the frequency of a photon to its energy. Does a photon fit better in the particle ...
... Chapter 23 Atomic Physics Lecture Questions: 1) What is a photon? Do all photons have the same energy? What is a quantum value? Give some examples of other quantum values besides the photon. Write the equation relating the frequency of a photon to its energy. Does a photon fit better in the particle ...
Einstein`s Miraculous Year
... frequency needed for the photoelectric effect to work. Every metal has a minimum ...
... frequency needed for the photoelectric effect to work. Every metal has a minimum ...
Document
... Quantum mechanics is a theory. It is our current “standard model” for describing the behavior of matter and energy at the smallest scales (photons, atoms, nuclei, quarks, gluons, leptons, …). Probability: a quantity that connects wave and particles. ...
... Quantum mechanics is a theory. It is our current “standard model” for describing the behavior of matter and energy at the smallest scales (photons, atoms, nuclei, quarks, gluons, leptons, …). Probability: a quantity that connects wave and particles. ...
CLASSICAL-QUANTUM CORRESPONDENCE AND WAVE PACKET SOLUTIONS OF THE DIRAC
... Communicated by Jean-Francois Ganghoffer Abstract. The idea of wave mechanics leads naturally to assume the well-known relation E = ~ω in the specific form H = ~W , where H is the classical Hamiltonian of a particle and W is the dispersion relation of the sought-for wave equation. We derive the expr ...
... Communicated by Jean-Francois Ganghoffer Abstract. The idea of wave mechanics leads naturally to assume the well-known relation E = ~ω in the specific form H = ~W , where H is the classical Hamiltonian of a particle and W is the dispersion relation of the sought-for wave equation. We derive the expr ...
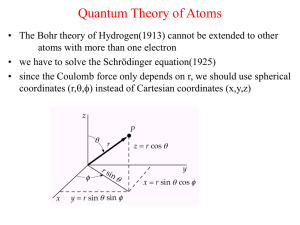
Chapter2. Elements of quantum mechanics
... particle-like behavior, then particles should be expected to show wave-like properties. de Broglie suggested that the wavelength of a particle is expressed as = h /p, where p is the momentum of a particle ...
... particle-like behavior, then particles should be expected to show wave-like properties. de Broglie suggested that the wavelength of a particle is expressed as = h /p, where p is the momentum of a particle ...
Quantum Mechanics Lecture 1 Dr. Mauro Ferreira
... By the end of the 19th century... Matter and radiation were described by Newtonian Mechanics and Maxwell’s equations, respectively Interaction between matter and radiation was explained by the Lorentz force Particle and wave properties were mutually exclusive ...
... By the end of the 19th century... Matter and radiation were described by Newtonian Mechanics and Maxwell’s equations, respectively Interaction between matter and radiation was explained by the Lorentz force Particle and wave properties were mutually exclusive ...
Wave as particle 2
... mass of two electrons ( 2me c 2 ) interact with the electric field of a nucleus, this photon may be turned into a pair of electron and positron. This process is called pair production through which energy gets turned into mass. Positron is the anti-particle of electron: it has the same mass as an el ...
... mass of two electrons ( 2me c 2 ) interact with the electric field of a nucleus, this photon may be turned into a pair of electron and positron. This process is called pair production through which energy gets turned into mass. Positron is the anti-particle of electron: it has the same mass as an el ...
File - SPHS Devil Physics
... a. The order of magnitude estimates from the uncertainty principle may include (but is not limited to) estimates of the energy of the ground state of an atom, the impossibility of an electron existing within a nucleus, and the lifetime of an electron in an excited energy state ...
... a. The order of magnitude estimates from the uncertainty principle may include (but is not limited to) estimates of the energy of the ground state of an atom, the impossibility of an electron existing within a nucleus, and the lifetime of an electron in an excited energy state ...
Torres: Copenhagen Quantum Mechanics
... “it becomes important to remember that science is concerned only with observable things and that we can observe an object only by letting it interact ...
... “it becomes important to remember that science is concerned only with observable things and that we can observe an object only by letting it interact ...