CHAPTER 2 INTRODUCTORYCHEMISTRY III. SAMPLE LECTURE
... 5. Hydrogen bonding provides temporary bonding between certain atoms within large complex molecules such as DNA. D. Chemical Reactions 1. A chemical reaction occurs when new bonds form and old bonds break between atoms. 2. SYNTHESIS REACTIONS occur when two or more atoms, ions, or molecules combine ...
... 5. Hydrogen bonding provides temporary bonding between certain atoms within large complex molecules such as DNA. D. Chemical Reactions 1. A chemical reaction occurs when new bonds form and old bonds break between atoms. 2. SYNTHESIS REACTIONS occur when two or more atoms, ions, or molecules combine ...
Ch8 sec4Life with Carbon
... Life with Carbon Chapter 8 Section 4 The four classes of organic compounds required by living things are carbohydrates, proteins, lipids and nucleic acids ...
... Life with Carbon Chapter 8 Section 4 The four classes of organic compounds required by living things are carbohydrates, proteins, lipids and nucleic acids ...
Body chemicals
... However, being present in small quantities does not mean an element is any less important for the functioning of a healthy body. Even in tiny amounts elements can have vital roles. Cobalt is an essential component of vitamin B12. Organic molecules Carbon atoms can bond to one another to form chains, ...
... However, being present in small quantities does not mean an element is any less important for the functioning of a healthy body. Even in tiny amounts elements can have vital roles. Cobalt is an essential component of vitamin B12. Organic molecules Carbon atoms can bond to one another to form chains, ...
Molecules of Life
... – Poly = ___________, meros = __________ – A large molecule that contains many molecules – A large molecule made of smaller, molecules of the same type (monomers) linked together. • A protein (the polymer) is made of many amino acids (monomers) ...
... – Poly = ___________, meros = __________ – A large molecule that contains many molecules – A large molecule made of smaller, molecules of the same type (monomers) linked together. • A protein (the polymer) is made of many amino acids (monomers) ...
File
... a. Fats (aka triglycerides) - most abundant in the body b. Phospholipids – contains phosphorus in addition to carbon, hydrogen and oxygen c. Steroids - contains cholesterol 1. Liver can manufacture cholesterol without a food source 2. Functions of Cholesterol o Essential in the structure of the semi ...
... a. Fats (aka triglycerides) - most abundant in the body b. Phospholipids – contains phosphorus in addition to carbon, hydrogen and oxygen c. Steroids - contains cholesterol 1. Liver can manufacture cholesterol without a food source 2. Functions of Cholesterol o Essential in the structure of the semi ...
Chapter 2, section 2
... different ways. • Some consumers get food by breaking down dead organisms or waste. They are • Decomposers ...
... different ways. • Some consumers get food by breaking down dead organisms or waste. They are • Decomposers ...
student-notes-copy-unit-review
... 1.) ________________________ compounds- Contain ______________ and __________________ atoms 2.) ________________________ compounds- Can have one or the other, but do not contain both carbon and hydrogen atoms A. Most of your body’s molecules are _______________________ compounds. a. ________________ ...
... 1.) ________________________ compounds- Contain ______________ and __________________ atoms 2.) ________________________ compounds- Can have one or the other, but do not contain both carbon and hydrogen atoms A. Most of your body’s molecules are _______________________ compounds. a. ________________ ...
Ch. 2 The Chemistry of Life
... - _____ contains the sugar _________, _____ contains the sugar _______________ ...
... - _____ contains the sugar _________, _____ contains the sugar _______________ ...
theory (casual usage of the word) vs. scientific theory
... is shown as a single point, the color representing the luminosity - this shows only those 66,976 our of 205,443 galaxies in the map that lie near the plane of Earth's equator. ...
... is shown as a single point, the color representing the luminosity - this shows only those 66,976 our of 205,443 galaxies in the map that lie near the plane of Earth's equator. ...
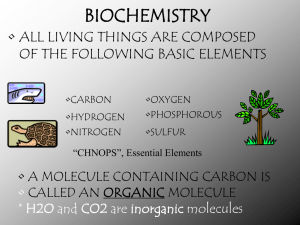
Carbon
... Specific heat refers to the amount of heat it takes to raise 1g, 1°C Water has a very high specific heat It takes a lot of energy to change the temperature of water, because of Hbonds ...
... Specific heat refers to the amount of heat it takes to raise 1g, 1°C Water has a very high specific heat It takes a lot of energy to change the temperature of water, because of Hbonds ...
Chemistry of Life notes
... Hydrolysis: a chemical reaction that takes in water (H2O) to break apart polymers monomers - Requires the addition of ONE water molecule. - this same reaction will be used to break down carbs, lipids, proteins and nucleic acids - Hydrolysis is the exact reverse of dehydration synthesis shown above ...
... Hydrolysis: a chemical reaction that takes in water (H2O) to break apart polymers monomers - Requires the addition of ONE water molecule. - this same reaction will be used to break down carbs, lipids, proteins and nucleic acids - Hydrolysis is the exact reverse of dehydration synthesis shown above ...
Chapter 3
... Top 6 most abundant elements in living things (not in order) * NCHOPS Top 8 elements in the earths crust (in order) * O, Si, Al, Fe (iron), Ca, Na (sodium), P, Mg Only silly apes in college study past midnight. ...
... Top 6 most abundant elements in living things (not in order) * NCHOPS Top 8 elements in the earths crust (in order) * O, Si, Al, Fe (iron), Ca, Na (sodium), P, Mg Only silly apes in college study past midnight. ...
Exam 1 Review KEY
... 14.) Define each of the structural levels of proteins. Primary – single strand of amino acids Secondary – 3D arrangement (alpha helix or beta sheet) Tertiary – overall molecular structure/secondary pieces that have come together Quaternary – 2+ polypeptides come together 15.) When DNA polymerase cre ...
... 14.) Define each of the structural levels of proteins. Primary – single strand of amino acids Secondary – 3D arrangement (alpha helix or beta sheet) Tertiary – overall molecular structure/secondary pieces that have come together Quaternary – 2+ polypeptides come together 15.) When DNA polymerase cre ...
Building Macromolecules Notes
... Class of macromolecules that do not dissolve in water Lipids usually serve one of three functions: ...
... Class of macromolecules that do not dissolve in water Lipids usually serve one of three functions: ...
Notes 3-3
... Similar to letters and words, the order of amino acids will determine which protein it is ...
... Similar to letters and words, the order of amino acids will determine which protein it is ...
Ch 3 Biochemistry Notes
... 3. Tertiary structure - results from interactions between amino acid side chains ...
... 3. Tertiary structure - results from interactions between amino acid side chains ...
Life`s Origin
... Hence concentrations of methane and hydrogen sulfide began to decrease, the ozone layer began to form. The rise of oxygen in the atmosphere drove some life forms to extinction. Some life forms evolved new, more efficient metabolic pathways that used oxygen for respiration others evolved as anaerobic ...
... Hence concentrations of methane and hydrogen sulfide began to decrease, the ozone layer began to form. The rise of oxygen in the atmosphere drove some life forms to extinction. Some life forms evolved new, more efficient metabolic pathways that used oxygen for respiration others evolved as anaerobic ...
Natural Selection - Madison County Schools
... Where did all the elements essential for life come from? How did they form into complex organisms? Chemical evolution refers to the formation of complex ORGANIC molecules from simple inorganic molecules through chemical reactions. This takes place in Earth’s oceans and lasts for less than a billion ...
... Where did all the elements essential for life come from? How did they form into complex organisms? Chemical evolution refers to the formation of complex ORGANIC molecules from simple inorganic molecules through chemical reactions. This takes place in Earth’s oceans and lasts for less than a billion ...
Abiogenesis

Abiogenesis (Brit.: /ˌeɪbaɪ.ɵˈdʒɛnɨsɪs/ AY-by-oh-JEN-ə-siss U.S. English pronunciation: /ˌeɪˌbaɪoʊˈdʒɛnᵻsɪs/), or biopoiesis, is the natural process of life arising from non-living matter, such as simple organic compounds. It is thought to have occurred on Earth between 3.8 and 4 billion years ago, and is studied through a combination of laboratory experiments and extrapolation from the genetic information of modern organisms in order to make reasonable conjectures about what pre-life chemical reactions may have given rise to a living system.The study of abiogenesis involves three main types of considerations: the geophysical, the chemical, and the biological, with more recent approaches attempting a synthesis of all three. Many approaches investigate how self-replicating molecules, or their components, came into existence. It is generally accepted that current life on Earth descended from an RNA world, although RNA-based life may not have been the first life to have existed. The Miller–Urey experiment and similar experiments demonstrated that most amino acids, basic chemicals of life, can be synthesized from inorganic compounds in conditions intended to be similar to early Earth. Several mechanisms have been investigated, including lightning and radiation. Other approaches (""metabolism first"" hypotheses) focus on understanding how catalysis in chemical systems in the early Earth might have provided the precursor molecules necessary for self-replication. Complex organic molecules have been found in the Solar System and in interstellar space, and these molecules may have provided starting material for the development of life on Earth.According to the panspermia hypothesis, microscopic life—distributed by meteoroids, asteroids and other small Solar System bodies—may exist throughout the Universe. It is speculated that the biochemistry of life may have begun shortly after the Big Bang, 13.8 billion years ago, during a habitable epoch when the age of the universe was only 10–17 million years.Nonetheless, Earth is the only place in the Universe known to harbor life. The age of the Earth is about 4.54 billion years. The earliest undisputed evidence of life on Earth dates at least from 3.5 billion years ago, during the Eoarchean Era after a geological crust started to solidify following the earlier molten Hadean Eon. There are microbial mat fossils found in 3.48 billion-year-old sandstone discovered in Western Australia. Other early physical evidence of a biogenic substance is graphite in 3.7 billion-year-old metasedimentary rocks discovered in southwestern Greenland.