thermodynamics
... 3. You attend a birthday party and cram an enormous amount of cake and ice cream into your stressed digestive system. You find out that this little snack amounted to over 1500 food Calories. To work this food off, how much equivalent mechanical work would you have to do (in Joules please)? (Note: 4. ...
... 3. You attend a birthday party and cram an enormous amount of cake and ice cream into your stressed digestive system. You find out that this little snack amounted to over 1500 food Calories. To work this food off, how much equivalent mechanical work would you have to do (in Joules please)? (Note: 4. ...
Chapters 12-15 Thermodynamics
... system to another system because one is hotter than the other • Heat flows from the hot system (lowering its internal energy and temperature) to a cold system (raising its internal energy and temperature). • Heat stops flowing when the two systems reach a common temperature. ...
... system to another system because one is hotter than the other • Heat flows from the hot system (lowering its internal energy and temperature) to a cold system (raising its internal energy and temperature). • Heat stops flowing when the two systems reach a common temperature. ...
Homework #1 Solutions
... 6c) None. In this case there is no piston to which the gas particles can trasfer their energy and do work on. Hence W = 0. 6d) None. The first law with W = 0 reads ∆U = Q. The properties of an ideal gas say that ∆U = 0 since temperature is held fixed. As a result Q = 0 too. Note that in a real gas, ...
... 6c) None. In this case there is no piston to which the gas particles can trasfer their energy and do work on. Hence W = 0. 6d) None. The first law with W = 0 reads ∆U = Q. The properties of an ideal gas say that ∆U = 0 since temperature is held fixed. As a result Q = 0 too. Note that in a real gas, ...
Schaums Heat
... What is the specific heat capacity of the metal? 7. A 200g copper calorimeter can contains 150 g of oil at 200C. To the oil is added 80 g of aluminum at 3000C. What will be the temperature of the system after equilibrium is established? 8. Exactly 3 g of carbon was burned to CO2 in a copper calorime ...
... What is the specific heat capacity of the metal? 7. A 200g copper calorimeter can contains 150 g of oil at 200C. To the oil is added 80 g of aluminum at 3000C. What will be the temperature of the system after equilibrium is established? 8. Exactly 3 g of carbon was burned to CO2 in a copper calorime ...
Calorimetry worksheet - MRS. STOTTS CHEMISTRY
... q means heat is released. + q means heat is absorbed. T is always final temperature – initial temperature. If something is getting hotter (10° 30°) the T is 30 – 10 = + 20°. (heat is absorbed) If something is getting cooler (75° 25°) the T is 25 – 75 = 50°. (heat is released) ...
... q means heat is released. + q means heat is absorbed. T is always final temperature – initial temperature. If something is getting hotter (10° 30°) the T is 30 – 10 = + 20°. (heat is absorbed) If something is getting cooler (75° 25°) the T is 25 – 75 = 50°. (heat is released) ...
Heat Transfer Powerpoint 1/6/15
... cooler molecules above them through direct contact. This convection current also pushes cooler molecules of water down to the bottom where they come in contact with the heated bottom of the teapot. While all of this is occurring, heat energy is being radiated in all directions from the from the burn ...
... cooler molecules above them through direct contact. This convection current also pushes cooler molecules of water down to the bottom where they come in contact with the heated bottom of the teapot. While all of this is occurring, heat energy is being radiated in all directions from the from the burn ...
Superconcepts
... a. Kinetic energy is the energy of objects in motion while potential energy is energy stored by position or in chemical bonds. b. In thermochemistry, the system is the chemical reaction itself (only the reactants & products). The surroundings are everything else in the universe. c. First law of ther ...
... a. Kinetic energy is the energy of objects in motion while potential energy is energy stored by position or in chemical bonds. b. In thermochemistry, the system is the chemical reaction itself (only the reactants & products). The surroundings are everything else in the universe. c. First law of ther ...
Thermochemistry - Lincoln
... Heat flows naturally from a hot object to a cold object; heat will not flow spontaneously from a cold object to a hot object. Understand that another statement of the Second Law is that no device is possible whose sole effect is to transform a given amount of heat completely into work. 12.11.68 Reco ...
... Heat flows naturally from a hot object to a cold object; heat will not flow spontaneously from a cold object to a hot object. Understand that another statement of the Second Law is that no device is possible whose sole effect is to transform a given amount of heat completely into work. 12.11.68 Reco ...
Current_Energy_Sustainable_Solutions
... Perspective: If I ride my bike 17 mph I can generate 100 Watts of power. If Everybody rode their bike for an 100 Watts then the world power would be 6.5 x 1011 W or .65 TW ...
... Perspective: If I ride my bike 17 mph I can generate 100 Watts of power. If Everybody rode their bike for an 100 Watts then the world power would be 6.5 x 1011 W or .65 TW ...
Energy
... Conservation of Energy The total amount of energy in a closed system remains constant over time (are said to be conserved over time) • The increase in the internal energy of a system is equal to the amount of energy added by heating the system minus the amount lost as a result of the work done by t ...
... Conservation of Energy The total amount of energy in a closed system remains constant over time (are said to be conserved over time) • The increase in the internal energy of a system is equal to the amount of energy added by heating the system minus the amount lost as a result of the work done by t ...
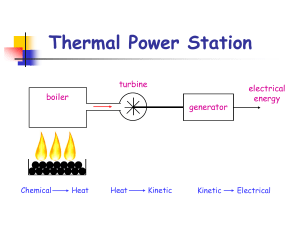
Thermal Power Station
... (b) The pipe delivers 1,280 kg of water to the turbine every second. Calculate the energy delivered to the power station in one second. ...
... (b) The pipe delivers 1,280 kg of water to the turbine every second. Calculate the energy delivered to the power station in one second. ...
Lessons 3 and 4 Thermodynamics
... Q = ΔU + W Q = The thermal energy given to a system (if this is negative, thermal energy is leaving the system) ...
... Q = ΔU + W Q = The thermal energy given to a system (if this is negative, thermal energy is leaving the system) ...
~therm= heat,temperature
... • An organism that has adapted to living in very high temperatures or heat, such as bacteria or algae ...
... • An organism that has adapted to living in very high temperatures or heat, such as bacteria or algae ...
problem-set-7c-cal-2016
... The specific heat of silver is 0.24 J/g . Co . How much heat needs to be added to a 184.2 g block of silver to get its temperature to rise 12.7 degrees? ...
... The specific heat of silver is 0.24 J/g . Co . How much heat needs to be added to a 184.2 g block of silver to get its temperature to rise 12.7 degrees? ...
Electronics Cooling MEP 635
... • The mechanism of heat transfer by radiation depends on the transfer of energy between surfaces by electromagnetic waves in the wave length interval between 0.1 to 100 μm. • Radiation heat transfer can travel in vacuum such as solar energy. • Radiation heat transfer depends on the surface propertie ...
... • The mechanism of heat transfer by radiation depends on the transfer of energy between surfaces by electromagnetic waves in the wave length interval between 0.1 to 100 μm. • Radiation heat transfer can travel in vacuum such as solar energy. • Radiation heat transfer depends on the surface propertie ...
Heat Transfer conduction
... the other. 2. Place the two cups in such a position that one end of the U-shaped aluminum bar can be inserted into the water in one cup, and the other end of the bar inserted into the water in the other cup. 3. Place a thermometer in each cup. 4. Record the temperature of each thermometer at 2 minut ...
... the other. 2. Place the two cups in such a position that one end of the U-shaped aluminum bar can be inserted into the water in one cup, and the other end of the bar inserted into the water in the other cup. 3. Place a thermometer in each cup. 4. Record the temperature of each thermometer at 2 minut ...
Chapter 7 Thermal and Energy Systems
... Calculate various energy, heat, work, and power quantities that are encountered in mechanical engineering, and express their numerical values in the SI and USCS. Describe how heat is transferred from one location to another by the processes of conduction, convection, and radiation. ...
... Calculate various energy, heat, work, and power quantities that are encountered in mechanical engineering, and express their numerical values in the SI and USCS. Describe how heat is transferred from one location to another by the processes of conduction, convection, and radiation. ...
SPECIFIC HEAT
... CALORIMETRY & SPECIFIC HEAT THEORY Heat energy is defined as energy that flows from hot objects to cold objects. It can be measured in calories, kilocalories, or joules of energy. One calorie is defined as the amount of heat required to raise the temperature of 1 gram of water by 1 C. One calorie i ...
... CALORIMETRY & SPECIFIC HEAT THEORY Heat energy is defined as energy that flows from hot objects to cold objects. It can be measured in calories, kilocalories, or joules of energy. One calorie is defined as the amount of heat required to raise the temperature of 1 gram of water by 1 C. One calorie i ...
Thermodynamics Guided Notes
... missed in class. It is very important to keep up with the reading and homework from this unit since we don’t have much time to cover it and because you’ll learn so much more that way! ...
... missed in class. It is very important to keep up with the reading and homework from this unit since we don’t have much time to cover it and because you’ll learn so much more that way! ...
Cogeneration

Cogeneration or combined heat and power (CHP) is the use of a heat engine or power station to generate electricity and useful heat at the same time. Trigeneration or combined cooling, heat and power (CCHP) refers to the simultaneous generation of electricity and useful heating and cooling from the combustion of a fuel or a solar heat collector. Cogeneration is a thermodynamically efficient use of fuel. In separate production of electricity, some energy must be discarded as waste heat, but in cogeneration this thermal energy is put to use. All thermal power plants emit heat during electricity generation, which can be released into the natural environment through cooling towers, flue gas, or by other means. In contrast, CHP captures some or all of the by-product for heating, either very close to the plant, or—especially in Scandinavia and Eastern Europe—as hot water for district heating with temperatures ranging from approximately 80 to 130 °C. This is also called combined heat and power district heating (CHPDH). Small CHP plants are an example of decentralized energy. By-product heat at moderate temperatures (100–180 °C, 212–356 °F) can also be used in absorption refrigerators for cooling.The supply of high-temperature heat first drives a gas or steam turbine-powered generator and the resulting low-temperature waste heat is then used for water or space heating as described in cogeneration. At smaller scales (typically below 1 MW) a gas engine or diesel engine may be used. Trigeneration differs from cogeneration in that the waste heat is used for both heating and cooling, typically in an absorption refrigerator. CCHP systems can attain higher overall efficiencies than cogeneration or traditional power plants. In the United States, the application of trigeneration in buildings is called building cooling, heating and power (BCHP). Heating and cooling output may operate concurrently or alternately depending on need and system construction.Cogeneration was practiced in some of the earliest installations of electrical generation. Before central stations distributed power, industries generating their own power used exhaust steam for process heating. Large office and apartment buildings, hotels and stores commonly generated their own power and used waste steam for building heat. Due to the high cost of early purchased power, these CHP operations continued for many years after utility electricity became available.