Specific and latent heat
... 2. What happens to the molecules when a solid changes to a liquid? 3. What happens to the molecules in a solid when it is heated? 4. What is meant by the specific heat capacity of a substance? 5. How much heat energy is needed to heat 4kg of aluminium by 80C? [Specific Heat Capacity of aluminium = 1 ...
... 2. What happens to the molecules when a solid changes to a liquid? 3. What happens to the molecules in a solid when it is heated? 4. What is meant by the specific heat capacity of a substance? 5. How much heat energy is needed to heat 4kg of aluminium by 80C? [Specific Heat Capacity of aluminium = 1 ...
Practice ws on Ch 5 - mvhs
... 1. How many joules must be added to a 63.43-g sample of Fe(s) to raise the sample’s temperature from 19.7 to 54.2° C? Assume that the specific heat of iron is constant over this temperature range. Answer: 9.8 x 10^2 J 2. How many joules must be added to a 7.92-g sample of CH3CH2OH(l) to raise the sa ...
... 1. How many joules must be added to a 63.43-g sample of Fe(s) to raise the sample’s temperature from 19.7 to 54.2° C? Assume that the specific heat of iron is constant over this temperature range. Answer: 9.8 x 10^2 J 2. How many joules must be added to a 7.92-g sample of CH3CH2OH(l) to raise the sa ...
Chapter 5, Problem 1
... Assumptions 1 Heat transfer is steady since there is no indication of any change with time. 2 Heat transfer is one-dimensional since the plate is large relative to its thickness, and there is thermal symmetry about the center plane 3 Thermal conductivity is constant. 4 Heat generation is uniform. Pr ...
... Assumptions 1 Heat transfer is steady since there is no indication of any change with time. 2 Heat transfer is one-dimensional since the plate is large relative to its thickness, and there is thermal symmetry about the center plane 3 Thermal conductivity is constant. 4 Heat generation is uniform. Pr ...
Heat Transfer: Conduction, Convection and Latent Heat In addition
... warmed by contact with the ground and moistened by evaporation ...
... warmed by contact with the ground and moistened by evaporation ...
Name
... a. Which has a higher enthalpy? Products or Reactants b. Was heat absorbed or released? c. Is this an endothermic or exothermic reaction? d. Is ΔH for this reaction positive or negative? e. Would the ΔH be on the left or right side of the yield sign? f. Is the reverse reaction exothermic or endother ...
... a. Which has a higher enthalpy? Products or Reactants b. Was heat absorbed or released? c. Is this an endothermic or exothermic reaction? d. Is ΔH for this reaction positive or negative? e. Would the ΔH be on the left or right side of the yield sign? f. Is the reverse reaction exothermic or endother ...
Lab 1: Temperature and Heat
... (a) Connect a 1.0 Ω resistor to a DC power supply, adjust the voltage to 5.0 V, and turn off the supply. (b) Add some liquid nitrogen to the insulated container and immerse the resistor. Once the resistor's temperature has dropped to liquid nitrogen temperature (77 K), measure the resistance of the ...
... (a) Connect a 1.0 Ω resistor to a DC power supply, adjust the voltage to 5.0 V, and turn off the supply. (b) Add some liquid nitrogen to the insulated container and immerse the resistor. Once the resistor's temperature has dropped to liquid nitrogen temperature (77 K), measure the resistance of the ...
IB 3.1, 3.2 Review
... Mastery quizzes are worth 10 points and will be two multiple choice questions with 8 points of problems. (Usually one or two problems). ...
... Mastery quizzes are worth 10 points and will be two multiple choice questions with 8 points of problems. (Usually one or two problems). ...
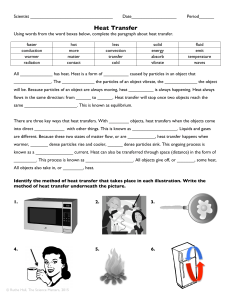
Heat Transfer - Granville County Public Schools
... same _____________________. This is known as equilibrium. There are three key ways that heat transfers. With ________ objects, heat transfers when the objects come into direct ____________ with other things. This is known as __________________. Liquids and gases are different. Because these two stat ...
... same _____________________. This is known as equilibrium. There are three key ways that heat transfers. With ________ objects, heat transfers when the objects come into direct ____________ with other things. This is known as __________________. Liquids and gases are different. Because these two stat ...
Γ = Γ ∙ (1)
... 2oF/1000 ft, leading to net cooling of around 3.50F/1000 ft. Equation (1) can be solved for every temperature that appears on a thermodynamic chart, resulting in a family of lines called moist adiabats. Since the moist adiabatic rate given by (1) is not constant, these lines are curves whose mean sl ...
... 2oF/1000 ft, leading to net cooling of around 3.50F/1000 ft. Equation (1) can be solved for every temperature that appears on a thermodynamic chart, resulting in a family of lines called moist adiabats. Since the moist adiabatic rate given by (1) is not constant, these lines are curves whose mean sl ...
Meltdown: Heat Conduction in Different Materials
... 5. Take a piece of straw and tape it tightly to the outside edge of each of the black blocks. Try to put the straw in the same place on each block. 6. Put the blocks down on a flat surface next to their labels—make sure you don’t mix them up! Place a black rubber O-ring in the middle of each block. ...
... 5. Take a piece of straw and tape it tightly to the outside edge of each of the black blocks. Try to put the straw in the same place on each block. 6. Put the blocks down on a flat surface next to their labels—make sure you don’t mix them up! Place a black rubber O-ring in the middle of each block. ...
Heat Energy Transfer
... • When heat is moved from one place to another without using particles, it is called radiation • Heat radiation is infrared energy waves ...
... • When heat is moved from one place to another without using particles, it is called radiation • Heat radiation is infrared energy waves ...
File
... groups or aggregates of molecules within fluids (e.g., liquids, gases) and rheids, either through advection or through diffusion or as a combination of both of them. Convection of mass cannot take place in solids, since neither bulk current flows nor significant diffusion can take place in solids. D ...
... groups or aggregates of molecules within fluids (e.g., liquids, gases) and rheids, either through advection or through diffusion or as a combination of both of them. Convection of mass cannot take place in solids, since neither bulk current flows nor significant diffusion can take place in solids. D ...
Introduction - UniMAP Portal
... Calculate the convection heat transfer through forced convection heat transfer inside pipe (dimensionless no.), heat transfer coefficient for laminar flow inside pipe, heat transfer coefficient for turbulent flow inside pipe, heat transfer coefficient for transition flow inside pipe, heat transfer c ...
... Calculate the convection heat transfer through forced convection heat transfer inside pipe (dimensionless no.), heat transfer coefficient for laminar flow inside pipe, heat transfer coefficient for turbulent flow inside pipe, heat transfer coefficient for transition flow inside pipe, heat transfer c ...
How long does it take to boil an egg?
... The heating of simple geometric objects dipped into an isothermal bath has been analysed under simple approximations in order to estimate the time evolution of the temperature. Our method succeeds in identifying an important dimensionless parameter, the Fourier modulus (equation (10)), which summari ...
... The heating of simple geometric objects dipped into an isothermal bath has been analysed under simple approximations in order to estimate the time evolution of the temperature. Our method succeeds in identifying an important dimensionless parameter, the Fourier modulus (equation (10)), which summari ...
Name - Net Start Class
... 5. What are the 2 units that can be used to measure heat? Joules and calories 6. A 1000 g piece of a metal at a temperature of 157oC is placed into a container of 2500 g of water at a temperature of 25oC. The final temperature of both the metal and the water is 45oC. a. What will happen to the tempe ...
... 5. What are the 2 units that can be used to measure heat? Joules and calories 6. A 1000 g piece of a metal at a temperature of 157oC is placed into a container of 2500 g of water at a temperature of 25oC. The final temperature of both the metal and the water is 45oC. a. What will happen to the tempe ...
Homework 3 Solutions ()
... Plugging these into the three-dimensional heat equation shows that T does, in fact, satisfy this equation. (b) (0.5 point) Describe what happens to the temperature of the body after a long period of time. Over long periods of time, we can think of what happens as t → ∞. In this case, we know that co ...
... Plugging these into the three-dimensional heat equation shows that T does, in fact, satisfy this equation. (b) (0.5 point) Describe what happens to the temperature of the body after a long period of time. Over long periods of time, we can think of what happens as t → ∞. In this case, we know that co ...
Midterm 3 - overview
... volume (½v2) and the potential energy per unit volume (gy) is constant at all points along a path of flow. Note that for an incompressible fluid: A1v1=A2v2 This is called the equation of continuity. ...
... volume (½v2) and the potential energy per unit volume (gy) is constant at all points along a path of flow. Note that for an incompressible fluid: A1v1=A2v2 This is called the equation of continuity. ...
Question Paper
... in the two rings of radii b and separation h. When the voltage is turned off the two sides (the capacitor C1 on one side and the rings on the other) are exactly balanced. Ignore wire resistance, inductance and gravitational effects. (a) Obtain an expression for the time-averaged force between the pl ...
... in the two rings of radii b and separation h. When the voltage is turned off the two sides (the capacitor C1 on one side and the rings on the other) are exactly balanced. Ignore wire resistance, inductance and gravitational effects. (a) Obtain an expression for the time-averaged force between the pl ...