Chapter 3
... However, we usually are not interested in the total energy needed or the total energy that can be recovered from a system. We will be more interested in the work involved in a system. For isothermal surroundings, the system can extract heat from the surroundings for free, so the work required to cre ...
... However, we usually are not interested in the total energy needed or the total energy that can be recovered from a system. We will be more interested in the work involved in a system. For isothermal surroundings, the system can extract heat from the surroundings for free, so the work required to cre ...
Thermal Stresses - Rick Bradford Home Page
... A body which is completely free can also give rise to thermal stress – but the thermal strains must be non-uniform. Thermal stresses can therefore arise in isotropic materials due to non-uniform temperature distributions. Alternatively, a uniform temperature change can result in thermal stresses if ...
... A body which is completely free can also give rise to thermal stress – but the thermal strains must be non-uniform. Thermal stresses can therefore arise in isotropic materials due to non-uniform temperature distributions. Alternatively, a uniform temperature change can result in thermal stresses if ...
Heat Transfer - cloudfront.net
... When each glob gets to the top, it falls back down. • What do you think is happening? • Is this a method of heat transfer? © Copyright 2015 – All rights reserved. www.cpalms.org ...
... When each glob gets to the top, it falls back down. • What do you think is happening? • Is this a method of heat transfer? © Copyright 2015 – All rights reserved. www.cpalms.org ...
Lesson Plan: What Makes Something Feel Warm? Modeling Energy
... Defroster for the Forgetful Chef”. In the lesson, students are asked to use their knowledge of materials to design a device that will defrost quickly. Background Information for Teachers Although most students have a common sense idea about what energy is, they may find it difficult to give a precis ...
... Defroster for the Forgetful Chef”. In the lesson, students are asked to use their knowledge of materials to design a device that will defrost quickly. Background Information for Teachers Although most students have a common sense idea about what energy is, they may find it difficult to give a precis ...
Lacture №1. Chemical thermodynamics. The first law of
... Process is the change of all or individual parameters of the system during the length of time (the period of time) Classification of a process according to the constant parameter of a system are: Isothermic process – temperature is constant, ...
... Process is the change of all or individual parameters of the system during the length of time (the period of time) Classification of a process according to the constant parameter of a system are: Isothermic process – temperature is constant, ...
chapter 5 thermochemistry
... Introduction and Section 5.1 Thermodynamics is the study of energy and its transformations. In this chapter we have focused on thermochemistry, the transformations of energy--especially heat--during chemical reactions. An object can possess energy in two forms: Kinetic energy is the energy due to mo ...
... Introduction and Section 5.1 Thermodynamics is the study of energy and its transformations. In this chapter we have focused on thermochemistry, the transformations of energy--especially heat--during chemical reactions. An object can possess energy in two forms: Kinetic energy is the energy due to mo ...
ISCI 2002 fall 2012 review test 2.tst
... 28) Earth and the Moon gravitationally attract each other. Does the more massive Earth attract the Moon with a greater force, the same force, or less force than the Moon attracts Earth? What reasoning guides your answer? 29) Explain why putting a dented Ping-Pong ball in a pot of boiling water will ...
... 28) Earth and the Moon gravitationally attract each other. Does the more massive Earth attract the Moon with a greater force, the same force, or less force than the Moon attracts Earth? What reasoning guides your answer? 29) Explain why putting a dented Ping-Pong ball in a pot of boiling water will ...
Optimal heating and cooling strategies for heat exchanger design
... The following extensive energy consumers are often equipped to exploit this available work: factories with cogeneration capabilities, distillation plants driven by heat pumps or with reuse of the process heat at lower temperatures, and the electric utility providing the energy, especially if it also ...
... The following extensive energy consumers are often equipped to exploit this available work: factories with cogeneration capabilities, distillation plants driven by heat pumps or with reuse of the process heat at lower temperatures, and the electric utility providing the energy, especially if it also ...
Thermal fluid flow through porous media containing obstacles
... many scientifique and engeeniring field. In this paper a numerical simulation was carried out for heat transfer and Fluid flow in a porous channel containing hot solid blocks having different geometries and located at different positions. This study, interested on the effect of parameters such as Re ...
... many scientifique and engeeniring field. In this paper a numerical simulation was carried out for heat transfer and Fluid flow in a porous channel containing hot solid blocks having different geometries and located at different positions. This study, interested on the effect of parameters such as Re ...
Change of state - Mrs. Coyle`s College Chemistry
... Molar heats of fusion are generally much smaller than molar heats of vaporization (liquid molecules are packed closer together and more energy need to rearrange from a solid to liquid) ...
... Molar heats of fusion are generally much smaller than molar heats of vaporization (liquid molecules are packed closer together and more energy need to rearrange from a solid to liquid) ...
Entransy Dissipation Analysis for Optimal Design of Heat Exchangers
... entransy by the analogy between heat and electrical conductions. The entransy of the new physical quantity can be used to optimize heat transfer processes. An analysis of the heat transfer from an object shows that the entransy, which corresponds to the electric potential energy in a capacitor, poss ...
... entransy by the analogy between heat and electrical conductions. The entransy of the new physical quantity can be used to optimize heat transfer processes. An analysis of the heat transfer from an object shows that the entransy, which corresponds to the electric potential energy in a capacitor, poss ...
Thermal Physics
... Microscopic explanation: A higher temperature means faster moving particles that collide with the walls more often and with greater force. However, if the volume of the gas is allowed to increase, the rate at which these particles hit the walls will decrease and thus the average force exerted on the ...
... Microscopic explanation: A higher temperature means faster moving particles that collide with the walls more often and with greater force. However, if the volume of the gas is allowed to increase, the rate at which these particles hit the walls will decrease and thus the average force exerted on the ...
ourse 228 File
... (2) Nitrogen at an initial state of 300K,150 kPa and 0.2 m3 is compressed by isothermal process to a final pressure of 800 kPa. Find work done. (3)What is difference between heat transfer and work transfer. (4)Show that for a closed system under going constant pressure process Qnet in-Wnet out=H2-H1 ...
... (2) Nitrogen at an initial state of 300K,150 kPa and 0.2 m3 is compressed by isothermal process to a final pressure of 800 kPa. Find work done. (3)What is difference between heat transfer and work transfer. (4)Show that for a closed system under going constant pressure process Qnet in-Wnet out=H2-H1 ...
Announcements
... the increase in entropy is equal to the amount of heat ◆ not a closed system added to the system divided ◆ energy input from the Sun ...
... the increase in entropy is equal to the amount of heat ◆ not a closed system added to the system divided ◆ energy input from the Sun ...
29-008-exam2
... the temperature does not change. • Heat of fusion: amount of energy required to melt 1 kg of material. • Heat of vaporization: amount of energy required to change 1 kg of material from liquid to vapor. ...
... the temperature does not change. • Heat of fusion: amount of energy required to melt 1 kg of material. • Heat of vaporization: amount of energy required to change 1 kg of material from liquid to vapor. ...
CHAPTER I
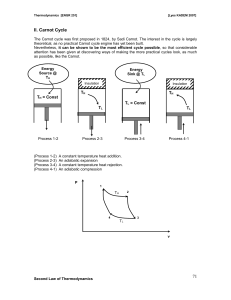
... 410 kJ for an energy input by heat transfer of 1000 kJ. The system undergoing the cycle receives the heat transfer from hot gases at a temperature of 500 K and discharges energy by heat transfer to the atmosphere at 300 K. Evaluate this claim. Example 2 When a fridge stands, in a room at 20°C, the m ...
... 410 kJ for an energy input by heat transfer of 1000 kJ. The system undergoing the cycle receives the heat transfer from hot gases at a temperature of 500 K and discharges energy by heat transfer to the atmosphere at 300 K. Evaluate this claim. Example 2 When a fridge stands, in a room at 20°C, the m ...