heat exchanger - Universitas Mercu Buana
... Heat exchangers may be divided into several categories or classifications. In the most commonly used type of heat exchanger, two fluids of different temperature flow in spaces separated by a tube wall. They transfer heat by convection and by conduction through the wall. This type is referred to as a ...
... Heat exchangers may be divided into several categories or classifications. In the most commonly used type of heat exchanger, two fluids of different temperature flow in spaces separated by a tube wall. They transfer heat by convection and by conduction through the wall. This type is referred to as a ...
Plants and Animals – Common Challenges
... Sweat gland Adrenal in chest wall expand as muscle in secretions gland contract more their wall relaxes; more increase; the secretions frequently; faster metabolic heat gets evaporation drop off; breathing speeds heat transfer from shunted to skin, where of sweat cools excitement it dissipates into ...
... Sweat gland Adrenal in chest wall expand as muscle in secretions gland contract more their wall relaxes; more increase; the secretions frequently; faster metabolic heat gets evaporation drop off; breathing speeds heat transfer from shunted to skin, where of sweat cools excitement it dissipates into ...
Global warming
... easy to bend the truth with statistics. Remember the Nuclear winter threat where greenhouse gases would make us freeze to death, or the y2k scare with its catastrophic consequences. Some politicians borrow doomsday science for their agenda and political gain. However, there is a silent group of scie ...
... easy to bend the truth with statistics. Remember the Nuclear winter threat where greenhouse gases would make us freeze to death, or the y2k scare with its catastrophic consequences. Some politicians borrow doomsday science for their agenda and political gain. However, there is a silent group of scie ...
Electrical Equivalent of Heat
... amount of water to cover entirely the heating coil; this water to have a temperature between 5 and 10 degrees below the temperature of the room. The heating coil, an ammeter and a battery (~ 6 Volt) should be put in series (see Figure 2) with a switch so that the circuit can be closed at a given ins ...
... amount of water to cover entirely the heating coil; this water to have a temperature between 5 and 10 degrees below the temperature of the room. The heating coil, an ammeter and a battery (~ 6 Volt) should be put in series (see Figure 2) with a switch so that the circuit can be closed at a given ins ...
Sec. 15.1 - Midland Park School District
... The specific heat of any substance is the amount of heat required to raise the temperature of 1 g of the substance by 1 0C. Recall that 1 calorie or 4.184 J is required to raise the temperature of one gram of pure water by 1 degree Celsius. The specific heat (C) of water, then, is 1cal or 4.184 J. g ...
... The specific heat of any substance is the amount of heat required to raise the temperature of 1 g of the substance by 1 0C. Recall that 1 calorie or 4.184 J is required to raise the temperature of one gram of pure water by 1 degree Celsius. The specific heat (C) of water, then, is 1cal or 4.184 J. g ...
Potential energy - Midland Park School District
... The specific heat of any substance is the amount of heat required to raise the temperature of 1 g of the substance by 1 0C. Recall that one calorie or 4.184 J is required to raise the temperature of one gram of pure water by 1 degree Celsius. The specific heat (C) of water, then, is 1cal or 4.184 J. ...
... The specific heat of any substance is the amount of heat required to raise the temperature of 1 g of the substance by 1 0C. Recall that one calorie or 4.184 J is required to raise the temperature of one gram of pure water by 1 degree Celsius. The specific heat (C) of water, then, is 1cal or 4.184 J. ...
Bacon¹s inductive method, example of heat.
... From the SEP: http://plato.stanford.edu/entries/francis-bacon/ “Forms, as the final result of the methodical procedure, are: nothing more than those laws and determinations of absolute actuality which govern and constitute any simple nature, as heat, light, weight, in every kind of matter and subjec ...
... From the SEP: http://plato.stanford.edu/entries/francis-bacon/ “Forms, as the final result of the methodical procedure, are: nothing more than those laws and determinations of absolute actuality which govern and constitute any simple nature, as heat, light, weight, in every kind of matter and subjec ...
E.ES.07.73 Fall 08
... time. 2. Specific Heat: is the amount of heat required to raise the temperature of an object 1 degree Celsius. 3. Global Warming: the increase in the average temperature of the Earth’s air and oceans. Ocean climate is driven by “climatic controls.” Climatic controls are permanent factors that drive ...
... time. 2. Specific Heat: is the amount of heat required to raise the temperature of an object 1 degree Celsius. 3. Global Warming: the increase in the average temperature of the Earth’s air and oceans. Ocean climate is driven by “climatic controls.” Climatic controls are permanent factors that drive ...
Understanding Heat Transfer, Conduction, Convection and Radiation
... • Heat Transfer: The transfer (passing) of heat from one object to another. Heat always moves in the direction from: higher temperatures to lower temperatures. warm to cool • Always! Always! Always from high energy to low! • Hot objects in a cooler room will cool to room temperature. • Cold objects ...
... • Heat Transfer: The transfer (passing) of heat from one object to another. Heat always moves in the direction from: higher temperatures to lower temperatures. warm to cool • Always! Always! Always from high energy to low! • Hot objects in a cooler room will cool to room temperature. • Cold objects ...
Thermodynamics test
... d) motor 23) What do you call an object that does not significantly change in temperature and internal energy even when heat is removed or added to it? a) Heat sink b) Reservoir c) Working substance d) Heat engine 24) The natural direction of heat flow is from high-temperature reservoir to a low tem ...
... d) motor 23) What do you call an object that does not significantly change in temperature and internal energy even when heat is removed or added to it? a) Heat sink b) Reservoir c) Working substance d) Heat engine 24) The natural direction of heat flow is from high-temperature reservoir to a low tem ...
Thermodynamics test
... 23) What do you call an object that does not significantly change in temperature and internal energy even when heat is removed or added to it? a) Heat sink b) Reservoir c) Working substance d) Heat engine 24) The natural direction of heat flow is from high-temperature reservoir to a low temperature ...
... 23) What do you call an object that does not significantly change in temperature and internal energy even when heat is removed or added to it? a) Heat sink b) Reservoir c) Working substance d) Heat engine 24) The natural direction of heat flow is from high-temperature reservoir to a low temperature ...
honors chem chpt 16 thermo
... Enthalpy change is the amount of energy absorbed by a system as heat during a process at constant pressure. H, change in enthalpy. Enthalpy for a reaction ...
... Enthalpy change is the amount of energy absorbed by a system as heat during a process at constant pressure. H, change in enthalpy. Enthalpy for a reaction ...
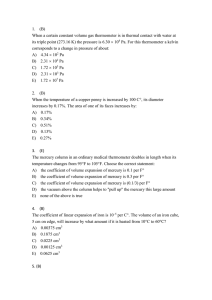
L 17 - Thermodynamics [2] Thermal Expansion Coefficients of linear
... • a thermocouple is placed in the pilot light • as long as the pilot light is on, the thermocouple is hot and current flows • a circuit senses the current and allows the main gas valve to open • if the pilot light is out, the circuit prevents the main gas valve from opening ...
... • a thermocouple is placed in the pilot light • as long as the pilot light is on, the thermocouple is hot and current flows • a circuit senses the current and allows the main gas valve to open • if the pilot light is out, the circuit prevents the main gas valve from opening ...
Homework 3
... dW = work done by the system dU = change in internal energy of the system The choices are a combination of adding or removing heat from a system and the compression or expansion of a system. With the above definition, when we add heat to the system, dQ is positive. Conversely dQ is negative when hea ...
... dW = work done by the system dU = change in internal energy of the system The choices are a combination of adding or removing heat from a system and the compression or expansion of a system. With the above definition, when we add heat to the system, dQ is positive. Conversely dQ is negative when hea ...
Entropy - Dordt College Homepages
... ice melting @ T = 0C Swater > Sice water more disordered ice melting - Sice > 0 (good) ...
... ice melting @ T = 0C Swater > Sice water more disordered ice melting - Sice > 0 (good) ...
U / ∂V
... temperature of a substance is determined by the quantity of the caloric gas which it contains; it was also assumed that the amount of caloric per unit mass is less for smaller particles than larger particles. These two assumptions explain the flow of the heat from the bodies with higher temperature ...
... temperature of a substance is determined by the quantity of the caloric gas which it contains; it was also assumed that the amount of caloric per unit mass is less for smaller particles than larger particles. These two assumptions explain the flow of the heat from the bodies with higher temperature ...
Document
... do not coincide with the months receiving the most or lowest radiation. The other processes which also control temperature (i.e. winds and surface ocean currents do not happen instantaneously. There is a “lag time”. ...
... do not coincide with the months receiving the most or lowest radiation. The other processes which also control temperature (i.e. winds and surface ocean currents do not happen instantaneously. There is a “lag time”. ...
Nervous tissue
... 5. What is homeostasis & how is it achieved? 6. What are the 2 types of thermoregulation? 7. How do organisms exchange heat with their environment? 8. How can organisms exchange heat within their bodies? 9. How do we achieve homeostasis for body temperature? 10. How do animals thermoregulate in temp ...
... 5. What is homeostasis & how is it achieved? 6. What are the 2 types of thermoregulation? 7. How do organisms exchange heat with their environment? 8. How can organisms exchange heat within their bodies? 9. How do we achieve homeostasis for body temperature? 10. How do animals thermoregulate in temp ...
Section 5.3
... • The Sun’s heat reaches Earth by a heat transfer process called radiation. • Once heat has arrived on Earth, there are three ways that it moves through the atmosphere: radiation, convection, and conduction. ...
... • The Sun’s heat reaches Earth by a heat transfer process called radiation. • Once heat has arrived on Earth, there are three ways that it moves through the atmosphere: radiation, convection, and conduction. ...
EXCRETION
... 4) makes urea from excess amino acids. 1. deamination the amine group (NH2) + an extra Hydrogen are removed from amino acids (a.a. can not be stored in the body) and converted to ammonia which is later converted into urea ...
... 4) makes urea from excess amino acids. 1. deamination the amine group (NH2) + an extra Hydrogen are removed from amino acids (a.a. can not be stored in the body) and converted to ammonia which is later converted into urea ...
Fluids and Thermodynamic Review BCE AAB DCD BDB CBE CEA
... (a) it increases (b) it remains constant (c) it decreases (d) it may increase or decrease depending on the shape of the rock 29. Salt water is denser than fresh water. A ship floats in both fresh water and salt water, the amount of water displaced by the salt water is (a) more (b) less (c) the same ...
... (a) it increases (b) it remains constant (c) it decreases (d) it may increase or decrease depending on the shape of the rock 29. Salt water is denser than fresh water. A ship floats in both fresh water and salt water, the amount of water displaced by the salt water is (a) more (b) less (c) the same ...
PS#3
... 3. A real gas showing a heating effect above its Joule-Thomson inversion temperature will show a cooling effect, no effect, heating effect below this inversion temperature. 11. A gas obeying the equation of state PV b RT undergoes a Joule-Thomson expansion. Show that as the pressure drops duri ...
... 3. A real gas showing a heating effect above its Joule-Thomson inversion temperature will show a cooling effect, no effect, heating effect below this inversion temperature. 11. A gas obeying the equation of state PV b RT undergoes a Joule-Thomson expansion. Show that as the pressure drops duri ...
Quiz_MATH.rtf
... A certain humidifier operates by raising water to the boiling point and then evaporating it. Every minute 30 g of water at 20C are added to replace the 30 g that are evaporated. The heat of fusion of water is333 kJ/kg, the heat of vaporization is 2256 kj/kg, and the specific heat is 4190 J/kg ...
... A certain humidifier operates by raising water to the boiling point and then evaporating it. Every minute 30 g of water at 20C are added to replace the 30 g that are evaporated. The heat of fusion of water is333 kJ/kg, the heat of vaporization is 2256 kj/kg, and the specific heat is 4190 J/kg ...
Hyperthermia
Hyperthermia is elevated body temperature due to failed thermoregulation that occurs when a body produces or absorbs more heat than it dissipates. Extreme temperature elevation then becomes a medical emergency requiring immediate treatment to prevent disability or death.The most common causes include heat stroke and adverse reactions to drugs. The former is an acute temperature elevation caused by exposure to excessive heat, or combination of heat and humidity, that overwhelms the heat-regulating mechanisms. The latter is a relatively rare side effect of many drugs, particularly those that affect the central nervous system. Malignant hyperthermia is a rare complication of some types of general anesthesia.Hyperthermia differs from fever in that the body's temperature set point remains unchanged. The opposite is hypothermia, which occurs when the temperature drops below that required to maintain normal metabolism.











![L 17 - Thermodynamics [2] Thermal Expansion Coefficients of linear](http://s1.studyres.com/store/data/014728078_1-e88e92f3857e030978e2ede6a9072797-300x300.png)