1-BUTANESULFONIC ACID SODIUM SALT
... DUDLEY CORPORATION provides the information herein in good faith but makes no representation as to its comprehensiveness or accuracy. This document is intended only as a guide to the appropriate precautionary handling of the material by a properly trained person using this. Individuals receiving the ...
... DUDLEY CORPORATION provides the information herein in good faith but makes no representation as to its comprehensiveness or accuracy. This document is intended only as a guide to the appropriate precautionary handling of the material by a properly trained person using this. Individuals receiving the ...
Unit B Chemistry Unit study guide
... How to determine chemical symbol and chemical formula (element vs compound) (atom vs molecule) What is chemical change and evidence of chemical change What to consider when choosing materials Product life cycle What happens with an Exothermic reaction Identify Number of elements and number of atoms ...
... How to determine chemical symbol and chemical formula (element vs compound) (atom vs molecule) What is chemical change and evidence of chemical change What to consider when choosing materials Product life cycle What happens with an Exothermic reaction Identify Number of elements and number of atoms ...
C6-Chemical Reactions
... heated, and relatively strong, especially when mixed with carbon in steel. Over time, objects made of iron will rust if they are left exposed to air. Rusting is a chemical change. You will learn to recognize chemical changes and to distinguish them from physical changes in this section. ...
... heated, and relatively strong, especially when mixed with carbon in steel. Over time, objects made of iron will rust if they are left exposed to air. Rusting is a chemical change. You will learn to recognize chemical changes and to distinguish them from physical changes in this section. ...
Unit 1 Matter Day 32 2016 Counting Atoms
... may be different from the reactants, the overall mass doesn’t change. ...
... may be different from the reactants, the overall mass doesn’t change. ...
CLASSROOM CONNECTORS
... Chemical properties are those properties a substance possesses because of its action or lack of action with other substances. Reaction with an acid, or reaction with oxygen (combustion) are just a couple of examples of chemical properties. Studying chemical properties is usually done when chemical c ...
... Chemical properties are those properties a substance possesses because of its action or lack of action with other substances. Reaction with an acid, or reaction with oxygen (combustion) are just a couple of examples of chemical properties. Studying chemical properties is usually done when chemical c ...
aspartic acid - West Liberty University
... where dust or mist is present, to the maximum use specified by the respirator supplier, NIOSH/OSHA approved half- face high-efficiency dust/mist filter respirator, or NIOSH/OSHA approved full face-piece high-efficiency dust/mist filter respirator. Higher or unknown concentrations, or for fire or spi ...
... where dust or mist is present, to the maximum use specified by the respirator supplier, NIOSH/OSHA approved half- face high-efficiency dust/mist filter respirator, or NIOSH/OSHA approved full face-piece high-efficiency dust/mist filter respirator. Higher or unknown concentrations, or for fire or spi ...
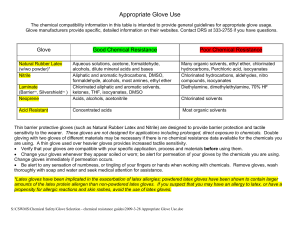
Appropriate Glove Use
... Chlorinated hydrocarbons, aldehydes, nitro compounds, isocyanates Diethylamine, dimethylethylamine, 70% HF ...
... Chlorinated hydrocarbons, aldehydes, nitro compounds, isocyanates Diethylamine, dimethylethylamine, 70% HF ...
Material Safety Data Sheet
... This product has been classified in accordance with the hazard criteria of the Controlled Products Regulations and the MSDS contains all of the information required by those regulations. Canadian Ingredient Disclosure List ...
... This product has been classified in accordance with the hazard criteria of the Controlled Products Regulations and the MSDS contains all of the information required by those regulations. Canadian Ingredient Disclosure List ...
Role of Chemistry in Everyday Life
... Drugs can be classified mainly on criteria outlined as follows: (a) On the basis of pharmacological effect This classification is based on pharmacological effect of the drugs. It is useful for doctors because it provides them the whole range of drugs available for the treatment of a particular type ...
... Drugs can be classified mainly on criteria outlined as follows: (a) On the basis of pharmacological effect This classification is based on pharmacological effect of the drugs. It is useful for doctors because it provides them the whole range of drugs available for the treatment of a particular type ...
81 Chemicals, Environment and Fertility
... an accumulative effect and create havoc with our health and fertility. This might explain why some older women have problems conceiving, because of the continuous contamination accumulated in body fat up to that point in her lifetime. Car exhaust fumes are alleged to cause infertility because they e ...
... an accumulative effect and create havoc with our health and fertility. This might explain why some older women have problems conceiving, because of the continuous contamination accumulated in body fat up to that point in her lifetime. Car exhaust fumes are alleged to cause infertility because they e ...
chemical reaction
... Chemical reactions are described by chemical equations. A chemical equation represents, with symbols and formulas, the identities and relative molecular or molar amounts of the reactants and products in a chemical reaction. For example, the following chemical equation shows that the reactant ammoni ...
... Chemical reactions are described by chemical equations. A chemical equation represents, with symbols and formulas, the identities and relative molecular or molar amounts of the reactants and products in a chemical reaction. For example, the following chemical equation shows that the reactant ammoni ...
chemical reaction - MRS. STOTTS CHEMISTRY
... Double-Displacement Reactions In double-displacement reactions, the ions of two compounds exchange places in an aqueous solution to form two new compounds. One of the compounds formed is usually a precipitate, an insoluble gas that bubbles out of the solution, or a molecular compound, usually ...
... Double-Displacement Reactions In double-displacement reactions, the ions of two compounds exchange places in an aqueous solution to form two new compounds. One of the compounds formed is usually a precipitate, an insoluble gas that bubbles out of the solution, or a molecular compound, usually ...
History of biotechnology Biotechnology is not something new but
... Equipment Hardware, bioreactors, software and consumables supporting biotechnology The growth in awareness of modern biotechnology parallels the serious world-wide changes in the economic climate arising from the escalation of oil prices since 1973. There is a growing realization that fossil fuels a ...
... Equipment Hardware, bioreactors, software and consumables supporting biotechnology The growth in awareness of modern biotechnology parallels the serious world-wide changes in the economic climate arising from the escalation of oil prices since 1973. There is a growing realization that fossil fuels a ...
Student Activity PDF - TI Education
... An integer immediately following a letter or closing parenthesis is converted to subscript. This is the number of atoms or group of atoms in a molecule. ...
... An integer immediately following a letter or closing parenthesis is converted to subscript. This is the number of atoms or group of atoms in a molecule. ...
Chemical Equations
... Don’t forget that the diatomic elements always have a subscript of two if not combined with another element. 2. Write the skeleton equation using formulas of reactants and products. Don’t forget the arrow between reactants and products. 3. Balance the equation by determining coefficients that provid ...
... Don’t forget that the diatomic elements always have a subscript of two if not combined with another element. 2. Write the skeleton equation using formulas of reactants and products. Don’t forget the arrow between reactants and products. 3. Balance the equation by determining coefficients that provid ...
Profile - Chimney Fabricators, Erection Contractor, Erection of
... The designs could include limpet coils & agitators like anchor, paddle, propeller or turbine type etc. as per customer requirement. ...
... The designs could include limpet coils & agitators like anchor, paddle, propeller or turbine type etc. as per customer requirement. ...
Ch. 6: Chemical Reactions Study Guide
... In a chemical reaction atoms are rearranged. A change of color is a sign that a chemical reaction is taking place. The changes that are visible during a chemical reaction are signs that the atoms in the reactants have been rearranged. A substance is said to be reduced when it gains electrons. A sign ...
... In a chemical reaction atoms are rearranged. A change of color is a sign that a chemical reaction is taking place. The changes that are visible during a chemical reaction are signs that the atoms in the reactants have been rearranged. A substance is said to be reduced when it gains electrons. A sign ...
in a Chemical Reactor - Max-Planck
... One of the reactors stands a good three meters high, yet it is thin enough for one person to encircle it with both arms. Temperature and pressure sensors reach into the reaction chamber with placement intervals of a hand’s width apart. The actual reaction chamber, well wrapped in insulating material ...
... One of the reactors stands a good three meters high, yet it is thin enough for one person to encircle it with both arms. Temperature and pressure sensors reach into the reaction chamber with placement intervals of a hand’s width apart. The actual reaction chamber, well wrapped in insulating material ...
Discover Chemical Changes - gk-12
... with before and after chemical changes so that students can make the chemical changes happen themselves or at least make observations of chemical changes that have happened at each station. I have listed above 9 possible chemical changes that can be used. Students should move from station to station ...
... with before and after chemical changes so that students can make the chemical changes happen themselves or at least make observations of chemical changes that have happened at each station. I have listed above 9 possible chemical changes that can be used. Students should move from station to station ...
How to Balance Chemical Equations
... atoms match from both sides. Each time any coefficient is adjusted, re-do the atom inventory on that side of the chemical equation. Repeat the process until total number of atoms for each element perfectly matches on both sides of the chemical equation. ...
... atoms match from both sides. Each time any coefficient is adjusted, re-do the atom inventory on that side of the chemical equation. Repeat the process until total number of atoms for each element perfectly matches on both sides of the chemical equation. ...
File - Flipped Out Science with Mrs. Thomas!
... kool-aid in water. It is an expected color change. It is a chemical change only when it is unexpected- for example mixing two clear liquids and having the substance turn blue. ...
... kool-aid in water. It is an expected color change. It is a chemical change only when it is unexpected- for example mixing two clear liquids and having the substance turn blue. ...
CH 11 Chemical Reaction WS #2 (Pre
... 1. What is the Great Barrier Reef and how was it formed? 2. Define chemical reaction3. How is a chemical reaction different from a physical one? Provide examples to support your explanation. 4. Explain how the appearance of the Statue of Liberty is an example of a chemical reaction: 5. What are stal ...
... 1. What is the Great Barrier Reef and how was it formed? 2. Define chemical reaction3. How is a chemical reaction different from a physical one? Provide examples to support your explanation. 4. Explain how the appearance of the Statue of Liberty is an example of a chemical reaction: 5. What are stal ...
Balancing Chemical Equations
... a. coefficients are numbers that go in front of the chemical formula b. 3 FeCl2 + 2 Al the 3 and 2 are coefficients.. ...
... a. coefficients are numbers that go in front of the chemical formula b. 3 FeCl2 + 2 Al the 3 and 2 are coefficients.. ...
Fine chemical

Fine chemicals are complex, single, pure chemical substances, produced in limited quantities in multipurpose plants by multistep batch chemical or biotechnological processes. They are described by exacting specifications, used for further processing within the chemical industry and sold for more than $10/kg (see the comparison of fine chemicals, commodities and specialties). The class of fine chemicals is subdivided either on the basis of the added value (building blocks, advanced intermediates or active ingredients), or the type of business transaction, namely standard or exclusive products.Fine chemicals are produced in limited volumes (< 1000 tons/year) and at relatively high prices (> $10/kg) according to exacting specifications, mainly by traditional organic synthesis in multipurpose chemical plants. Biotechnical processes are gaining ground. The global production value is about $85 billion. Fine chemicals are used as starting materials for specialty chemicals, particularly pharmaceuticals, biopharmaceuticals and agrochemicals. Custom manufacturing for the life science industry plays a big role; however, a significant portion of the fine chemicals total production volume is manufactured in house by large users. The industry is fragmented and extends from small, privately owned companies to divisions of big, diversified chemical enterprises. The term ""fine chemicals"" is used in distinction to ""heavy chemicals"", which are produced and handled in large lots and are often in a crude state.Since their inception in the late 1970s, fine chemicals have become an important part of the chemical industry. The total production value of $85 billion is split about 60 / 40 among in-house production by the main consumers, the life science industry, on the one hand, and the fine chemicals industry on the other hand. The latter pursues both a “supply push” strategy, whereby standard products are developed in-house and offered ubiquitously, and a “demand pull” strategy, whereby products or services determined by the customer are provided exclusively on a “one customer / one supplier” basis. The products are mainly used as building blocks for proprietary products. The hardware of the top tier fine chemical companies has become almost identical. The design, lay-out and equipment of the plants and laboratories has become practically the same all over the world. Most chemical reactions performed go back to the days of the dyestuff industry. Numerous regulations determine the way labs and plants have to be operated, thereby contributing to the uniformity.