Chemical Equilibrium - local.brookings.k12.sd.us
... products are in the same ________ physical _____ state are ____________, homogeneous but reactions with _________ reactants and ________ products in _____ more than ___ one ________ physical ________ _____ state result in _____________ heterogeneous gaseous _________ equilibria ethanol 1C2H5OH (l) K ...
... products are in the same ________ physical _____ state are ____________, homogeneous but reactions with _________ reactants and ________ products in _____ more than ___ one ________ physical ________ _____ state result in _____________ heterogeneous gaseous _________ equilibria ethanol 1C2H5OH (l) K ...
Covert Chemical... 2_Couvertures English chimie 4
... Chemical Reactions 2: Equilibrium and Oxidation-reduction is the third of the three Learning Guides for the Secondary V Chemistry program, which comprises the following three courses: Gases Chemical Reactions 1: Energy and Chemical Dynamics Chemical Reactions 2: Equilibrium and Oxidation-reduction ...
... Chemical Reactions 2: Equilibrium and Oxidation-reduction is the third of the three Learning Guides for the Secondary V Chemistry program, which comprises the following three courses: Gases Chemical Reactions 1: Energy and Chemical Dynamics Chemical Reactions 2: Equilibrium and Oxidation-reduction ...
National German Competition and Problems of the IChO
... best fit straight line (your results in integers again). Find an order of increasing polarity of all 13 solvents. f) ...
... best fit straight line (your results in integers again). Find an order of increasing polarity of all 13 solvents. f) ...
Silicic magma reservoirs in the Earth`s crust
... al. (2007b), Lipman (2007), Cashman and Sparks (2013), Cashman and Giordano (2014), and de Silva and Gregg (2014)]. At this stage, it is clear that magmas are complex mixtures of multiple phases (silicate melt, H2O-CO2-dominated fluid, and up to 10 different mineral phases in some systems) with very ...
... al. (2007b), Lipman (2007), Cashman and Sparks (2013), Cashman and Giordano (2014), and de Silva and Gregg (2014)]. At this stage, it is clear that magmas are complex mixtures of multiple phases (silicate melt, H2O-CO2-dominated fluid, and up to 10 different mineral phases in some systems) with very ...
National German competition
... d) Calculate the concentration of SO2(aq) in a solution of sodium hydrogensulfite (c = 0.01 molL-1) with the equilibrium constant of c). Drops of bromine are added to a solution of sulfur dioxide (c = 0.01 molL-1) until there is an excess of bromine. The total amount of sulfur dioxide is oxidized to ...
... d) Calculate the concentration of SO2(aq) in a solution of sodium hydrogensulfite (c = 0.01 molL-1) with the equilibrium constant of c). Drops of bromine are added to a solution of sulfur dioxide (c = 0.01 molL-1) until there is an excess of bromine. The total amount of sulfur dioxide is oxidized to ...
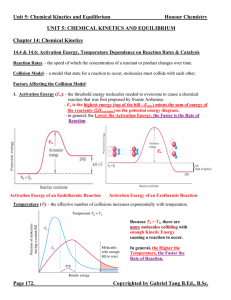
Unit 5: Chemical Kinetics and Equilibrium
... - the equilibrium state is dynamic (not static). Chemical species are continuously converting from reactants to products and vice versa. It appears that the reaction has stopped only because the rate of consumption = rate of production. - if an equilibrium state is disturbed (changing concentrations ...
... - the equilibrium state is dynamic (not static). Chemical species are continuously converting from reactants to products and vice versa. It appears that the reaction has stopped only because the rate of consumption = rate of production. - if an equilibrium state is disturbed (changing concentrations ...
Chemistry – A Molecular Sciences Appendices
... A compound is a pure substance that is made up of more than one element. Compounds can be ionic (CAMS Chapter 4) or covalent as described in (CAMS Chapter 5). Covalent compounds are said to be molecular because they exist as discrete molecules, but ionic compounds exist as extended three-dimensional ...
... A compound is a pure substance that is made up of more than one element. Compounds can be ionic (CAMS Chapter 4) or covalent as described in (CAMS Chapter 5). Covalent compounds are said to be molecular because they exist as discrete molecules, but ionic compounds exist as extended three-dimensional ...
Question Bank for Pre Board Exam(XII Chemistry)
... the formula of corundum. 38.Why is Frenkel defect not found in pure alkali metal halides? 39.Which point defect is observed in a crystal when a vacancy is created by an atom missing from a lattice site. 40. Why does conductivity of silicon increase with the rise in temperature? 41.Name the crystal d ...
... the formula of corundum. 38.Why is Frenkel defect not found in pure alkali metal halides? 39.Which point defect is observed in a crystal when a vacancy is created by an atom missing from a lattice site. 40. Why does conductivity of silicon increase with the rise in temperature? 41.Name the crystal d ...
1 Ag PO 7.5 10 1.79 10 418.57 mol x gL x M g
... Ksp values do not give highly accurate compound solubilities for a number of reasons including: (a) Ksp values are determined at a specific temperature and unless you use an accurately controlled temperature bath, the measured solubility will not be precisely what was calculated. (b) Ksp values assu ...
... Ksp values do not give highly accurate compound solubilities for a number of reasons including: (a) Ksp values are determined at a specific temperature and unless you use an accurately controlled temperature bath, the measured solubility will not be precisely what was calculated. (b) Ksp values assu ...
Entropy and Free Energy
... 14.3 to calculate the entropy change for a real process because of the difficulty involved in determining W, the number of different possible arrangements (Equation 14.2) in a macroscopic system. Instead, for processes other than isothermal expansion or compression of an ideal gas (for which we can ...
... 14.3 to calculate the entropy change for a real process because of the difficulty involved in determining W, the number of different possible arrangements (Equation 14.2) in a macroscopic system. Instead, for processes other than isothermal expansion or compression of an ideal gas (for which we can ...
The Skaergaard Layered Intrusion, East Greenland
... processes associated with magmatic flow and the latter refers to processes resulting from e.g. compaction and variations in rates of nucleation and crystallisation. There are three different series of layering present; the Layered Series that consists of magma that crystallised from the bottom upwar ...
... processes associated with magmatic flow and the latter refers to processes resulting from e.g. compaction and variations in rates of nucleation and crystallisation. There are three different series of layering present; the Layered Series that consists of magma that crystallised from the bottom upwar ...
AP Chem unit 13 presentation
... It is important to realize that although changes to the reaction may alter the equilibrium positions, they do not alter the equilibrium constant. ...
... It is important to realize that although changes to the reaction may alter the equilibrium positions, they do not alter the equilibrium constant. ...
Concept based notes Chemistry Lab Manual
... Q. 66. Why bromide and iodide do not show chromyl chloride test? Ans. Because chromyl bromide and chromyl iodide is unknown and not formed, instead of these bromine and chlorine are evolved. Q. 67. Describe the chemistry of match stick test? Ans. The sulphate is reduced to sulphide by carbon of matc ...
... Q. 66. Why bromide and iodide do not show chromyl chloride test? Ans. Because chromyl bromide and chromyl iodide is unknown and not formed, instead of these bromine and chlorine are evolved. Q. 67. Describe the chemistry of match stick test? Ans. The sulphate is reduced to sulphide by carbon of matc ...
Crystal structure of the simian immunodeficiency virus (SIV) gp41
... (HIV and SIV) mediates membrane fusion during viral entry. The crystal structure of the HIV-1 gp41 ectodomain core in its proposed fusion-active state is a six-helix bundle. Here we have reconstituted the core of the SIV gp41 ectodomain with two synthetic peptides called SIV N36 and SIV C34, which f ...
... (HIV and SIV) mediates membrane fusion during viral entry. The crystal structure of the HIV-1 gp41 ectodomain core in its proposed fusion-active state is a six-helix bundle. Here we have reconstituted the core of the SIV gp41 ectodomain with two synthetic peptides called SIV N36 and SIV C34, which f ...
Date: 16 / 01 / 2014 - Qatar University QSpace
... series of experiments were carried out to study the variation of preparation parameters such as support type, temperature, ‘ion-exchange’ time and the concentration of the (precursor) salt. ...
... series of experiments were carried out to study the variation of preparation parameters such as support type, temperature, ‘ion-exchange’ time and the concentration of the (precursor) salt. ...
1 General Chemistry II Jasperse Entropy, Spontaneity, and Free
... ΔSsurr < 0 and its magnitude is < ΔSsys. In other words, the system loses entropy and the surroundings also lose entropy. The loss by the surroundings is less than the loss by the system. ΔSsurr < 0 and its magnitude is > ΔSsys. In other words, the system loses entropy and the surroundings also lose ...
... ΔSsurr < 0 and its magnitude is < ΔSsys. In other words, the system loses entropy and the surroundings also lose entropy. The loss by the surroundings is less than the loss by the system. ΔSsurr < 0 and its magnitude is > ΔSsys. In other words, the system loses entropy and the surroundings also lose ...
Section – B - About iTutoring
... (61). What will be the sum of values of pH and pOH at 298 K temperature ? At 298 K, pH + pOH = 14. (62). On the basis of which values of pH, solutions can be said to be acidic, basic or neutral? pH = 7 neutral solution pH < 7 acidic solution pH > 7 basic solution (63). What is the pH of CH3COONa sol ...
... (61). What will be the sum of values of pH and pOH at 298 K temperature ? At 298 K, pH + pOH = 14. (62). On the basis of which values of pH, solutions can be said to be acidic, basic or neutral? pH = 7 neutral solution pH < 7 acidic solution pH > 7 basic solution (63). What is the pH of CH3COONa sol ...
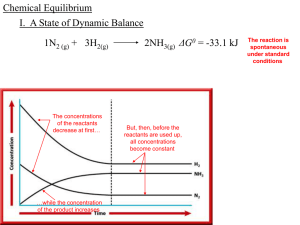
Chemical Equilibrium
... and pure liquids in Keq expressions. Only partial pressures for gas-phase substances or concentrations in solutions are included in the expressions of equilibrium constants. As such, the equilibrium constant expression for this reaction would simply be ...
... and pure liquids in Keq expressions. Only partial pressures for gas-phase substances or concentrations in solutions are included in the expressions of equilibrium constants. As such, the equilibrium constant expression for this reaction would simply be ...
Polarised Light in Science and Nature
... We humans cannot see when light is polarised and this leads us to unfortunate misapprehensions about it. Even scientists who should know better, often assume that polarised light is an obscure topic of specialised interest in only a few rather isolated areas; in fact it is a universal feature of our ...
... We humans cannot see when light is polarised and this leads us to unfortunate misapprehensions about it. Even scientists who should know better, often assume that polarised light is an obscure topic of specialised interest in only a few rather isolated areas; in fact it is a universal feature of our ...
Magma RheologyVariations in Sheet Intrusions of the
... solidified magma reservoir at depth. The inclusions preserve information on the fluid dynamics of the conduit systems, suggesting that the magma rheology of inclusionbearing segments changed from Newtonian to Binghamian upon entrainment. These potential internal variations in magma rheology, between ...
... solidified magma reservoir at depth. The inclusions preserve information on the fluid dynamics of the conduit systems, suggesting that the magma rheology of inclusionbearing segments changed from Newtonian to Binghamian upon entrainment. These potential internal variations in magma rheology, between ...
IIT-JEE (Advanced) - Brilliant Public School Sitamarhi
... If the amount of O2 evolved was 146.8 ml at S.T.P., calculate the % by weight of KClO4 in the residue. Q.13 A sample of calcium carbonate contains impurities which do not react with a mineral acid. When 2 grams of the sample were reacted with the mineral acid, 375 ml of carbon dioxide were obtained ...
... If the amount of O2 evolved was 146.8 ml at S.T.P., calculate the % by weight of KClO4 in the residue. Q.13 A sample of calcium carbonate contains impurities which do not react with a mineral acid. When 2 grams of the sample were reacted with the mineral acid, 375 ml of carbon dioxide were obtained ...
МЕТОДИЧЕСКИЕ УКАЗАНИЯ СТУДЕНТАМ
... 1. What volume (in mL) of 40% H3PO4 solution (ρ=1,25 g/cm3) is necessary to prepare 400mL of 0,25N of phosphoric acid solution (ρ=1g/cm3)? Calculate the mole fraction of H3PO4 in the obtained solution. 2. How many grams of Na2CO3·10H2O are necessary to prepare 100mL of 10% Na2CO3 solution (ρ=1,12 g/ ...
... 1. What volume (in mL) of 40% H3PO4 solution (ρ=1,25 g/cm3) is necessary to prepare 400mL of 0,25N of phosphoric acid solution (ρ=1g/cm3)? Calculate the mole fraction of H3PO4 in the obtained solution. 2. How many grams of Na2CO3·10H2O are necessary to prepare 100mL of 10% Na2CO3 solution (ρ=1,12 g/ ...
engineering chemistry
... An atom is the smallest form of a chemical particle that retains the properties of the particle. The word 'atom' comes from the Greek word 'atomos', meaning 'unable to be cut'. The original meaning of atom was the smallest, indivisible form of a chemical particle. Now we know how to divide atoms int ...
... An atom is the smallest form of a chemical particle that retains the properties of the particle. The word 'atom' comes from the Greek word 'atomos', meaning 'unable to be cut'. The original meaning of atom was the smallest, indivisible form of a chemical particle. Now we know how to divide atoms int ...
Problem 1-2 - IPN-Kiel
... v) Does the formation of Fe3O4 lead to a higher or to a lower calculated content of iron? Account for your answer. vi) Calculate the mass of the iron(III) chloride sample which was given into the measuring flask. ...
... v) Does the formation of Fe3O4 lead to a higher or to a lower calculated content of iron? Account for your answer. vi) Calculate the mass of the iron(III) chloride sample which was given into the measuring flask. ...
Curriculum Vitae - Université Paris-Sud
... by matter, including the non-homogeneous spatial distribution of initial ions and radicals, was better understood, at least in aqueous solutions. In particular, various metal ions were used widely as radical scavengers and redox indicators in the reduction or oxidation processes induced indirectly b ...
... by matter, including the non-homogeneous spatial distribution of initial ions and radicals, was better understood, at least in aqueous solutions. In particular, various metal ions were used widely as radical scavengers and redox indicators in the reduction or oxidation processes induced indirectly b ...
Crystallization

Crystallization is the (natural or artificial) process of formation of solid crystals precipitating from a solution, melt or more rarely deposited directly from a gas. Crystallization is also a chemical solid–liquid separation technique, in which mass transfer of a solute from the liquid solution to a pure solid crystalline phase occurs. In chemical engineering crystallization occurs in a crystallizer. Crystallization is therefore an aspect of precipitation, obtained through a variation of the solubility conditions of the solute in the solvent, as compared to precipitation due to chemical reaction.