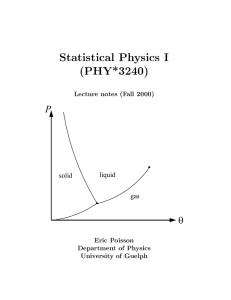
as PDF
... isothermal processes, respectively, with T1 < TH and TL < T2. There is also a heat loss Q from the hot reservoir to the cold reservoir and there are other internal irreversibilities (such as dissipative processes inside the working fluid). This Carnot-like model was chosen because of its simplicity ...
... isothermal processes, respectively, with T1 < TH and TL < T2. There is also a heat loss Q from the hot reservoir to the cold reservoir and there are other internal irreversibilities (such as dissipative processes inside the working fluid). This Carnot-like model was chosen because of its simplicity ...
PrOBLEMS_PACK
... The initial temperature and pressure are 17 C and 100 kPa, respectively, and the final pressure is 500 kPa. Assume Rs = 0.287 kJ/kgK and average specific heats cv = 0.723 kJ/kgK and cp = 1.01 kJ/kgK. Calculate: a) the final temperature in K; b) the work done on the gas, in kJ/kg; and c) the chan ...
... The initial temperature and pressure are 17 C and 100 kPa, respectively, and the final pressure is 500 kPa. Assume Rs = 0.287 kJ/kgK and average specific heats cv = 0.723 kJ/kgK and cp = 1.01 kJ/kgK. Calculate: a) the final temperature in K; b) the work done on the gas, in kJ/kg; and c) the chan ...
Chapter-9-Handouts
... Internal Energy (E): The capacity to do work or to produce heat. Temperature (T): How hot or cold an object is. Heat (q): The energy that is transferred as a result of a temperature difference between a system and its surroundings. ...
... Internal Energy (E): The capacity to do work or to produce heat. Temperature (T): How hot or cold an object is. Heat (q): The energy that is transferred as a result of a temperature difference between a system and its surroundings. ...
Optimizing natural gas fueling station reservoirs pressure based on
... At CNG fuelling station, natural gas is usually stored in a cascade storage system to utilize the station more efficient. The cascade storage system is generally divided into three reservoirs, commonly termed low, medium and high-pressure reservoirs. The pressures within these three reservoirs have ...
... At CNG fuelling station, natural gas is usually stored in a cascade storage system to utilize the station more efficient. The cascade storage system is generally divided into three reservoirs, commonly termed low, medium and high-pressure reservoirs. The pressures within these three reservoirs have ...
Chapter 6 NOTES!!!!! - Clinton Public Schools
... • When the temperature of an object increase, the average kinetic energy of the particles in the object increases. • Because thermal energy is the total kinetic and potential energy of all the particles in an object, the thermal energy of the object increases when the average kinetic energy of its p ...
... • When the temperature of an object increase, the average kinetic energy of the particles in the object increases. • Because thermal energy is the total kinetic and potential energy of all the particles in an object, the thermal energy of the object increases when the average kinetic energy of its p ...
Modern Industrial Assessment1
... 2. A high flue gas temperature often reflects the existence of deposits and fouling on the fire and/or water side(s) of the boiler. The resulting loss in boiler efficiency can be closely estimated on the basis that a 1-% efficiency loss occurs with every 400F increase in stack temperature. It is sug ...
... 2. A high flue gas temperature often reflects the existence of deposits and fouling on the fire and/or water side(s) of the boiler. The resulting loss in boiler efficiency can be closely estimated on the basis that a 1-% efficiency loss occurs with every 400F increase in stack temperature. It is sug ...
Thermodynamic Considerations in Animal Nutrition Department of
... budget of a population or trophic level comprises the sum of energy gains and losses by each individual organism. Since these energy exchanges are governed by the same thermodynamic principles that govern purely physical transfers, the animal energy budget can be developed according to the Thermodyn ...
... budget of a population or trophic level comprises the sum of energy gains and losses by each individual organism. Since these energy exchanges are governed by the same thermodynamic principles that govern purely physical transfers, the animal energy budget can be developed according to the Thermodyn ...
EDEXCEL HIGHERS ENGINEERING THERMODYNAMICS H2 NQF
... impacts with the surface and hence twice the pressure. To keep the pressure the same, the volume would have to be doubled or the temperature halved. It follows that the constant must contain the mass of the gas in order to reflect the number of molecules. The gas law can then be written as pV/T = mR ...
... impacts with the surface and hence twice the pressure. To keep the pressure the same, the volume would have to be doubled or the temperature halved. It follows that the constant must contain the mass of the gas in order to reflect the number of molecules. The gas law can then be written as pV/T = mR ...
Thermodynamic Cycles
... Irreversible Process An irreversible process cannot return both the system and the surroundings to their original conditions if reversed. For example, an automobile engine does not give back the fuel it took to drive up a hill as it coasts down the hill to its original position. There are factors th ...
... Irreversible Process An irreversible process cannot return both the system and the surroundings to their original conditions if reversed. For example, an automobile engine does not give back the fuel it took to drive up a hill as it coasts down the hill to its original position. There are factors th ...
WORK: Work is done when the force produces motion. Def: WORK is
... Electrical energy:the energy of moving electrons in a conductor(charged body ,electrical cell) Nuclear energy: The energy released when two nuclei of light elements combine to each other to form a heavy nucleus (called as nuclear fusion)or when the heavy nucleus breaks into two light nuclei .( calle ...
... Electrical energy:the energy of moving electrons in a conductor(charged body ,electrical cell) Nuclear energy: The energy released when two nuclei of light elements combine to each other to form a heavy nucleus (called as nuclear fusion)or when the heavy nucleus breaks into two light nuclei .( calle ...
Solutions Exercises Lecture 4
... First, the system is defined and all given data are collected. The system is isolated (no heat exchange between the system and the surroundings)and consists of A and B with a total mass of (mA + mB). The complete system is divided into two subsystems, namely A and B. Initially the two subsystems hav ...
... First, the system is defined and all given data are collected. The system is isolated (no heat exchange between the system and the surroundings)and consists of A and B with a total mass of (mA + mB). The complete system is divided into two subsystems, namely A and B. Initially the two subsystems hav ...
1 CHAPTER 17 CHEMICAL THERMODYNAMICS 17.1 Equilibrium
... Up to this point the thermodynamical systems that we have been considering have consisted of just a single component and, for the most part, just one phase, but we are now going to discuss systems consisting of more than one phase and more than one component. The Gibbs Phase Law provides a relation ...
... Up to this point the thermodynamical systems that we have been considering have consisted of just a single component and, for the most part, just one phase, but we are now going to discuss systems consisting of more than one phase and more than one component. The Gibbs Phase Law provides a relation ...
Energy Content from the Frameworks
... When matter undergoes change, it always involves energy moving into or out of the system, often in the form of heat. When heat energy is added, it changes the density of the air by adding energy to the molecules. Explain that this is the same principle that causes the formation of wind and ocean cur ...
... When matter undergoes change, it always involves energy moving into or out of the system, often in the form of heat. When heat energy is added, it changes the density of the air by adding energy to the molecules. Explain that this is the same principle that causes the formation of wind and ocean cur ...
The Laws of Thermodynamics - FSM-UKSW
... of all the molecules of a system — to energy transfers due to heat and work. The first law is universally valid, applicable to all kinds of processes, providing a connection between the microscopic and macroscopic worlds. There are two ways energy can be transferred between a system and its surround ...
... of all the molecules of a system — to energy transfers due to heat and work. The first law is universally valid, applicable to all kinds of processes, providing a connection between the microscopic and macroscopic worlds. There are two ways energy can be transferred between a system and its surround ...