periodic trend
... -Electrons are in higher energy levels as you move down a group; they are farther away from the positive “pull” of the nucleus and therefore easier to remove. ...
... -Electrons are in higher energy levels as you move down a group; they are farther away from the positive “pull” of the nucleus and therefore easier to remove. ...
Int. Sci. 9 Modern Periodic Table Powerpoint
... – Members of a group in the periodic table have similar chemical properties Known as Periodic Law!!! ...
... – Members of a group in the periodic table have similar chemical properties Known as Periodic Law!!! ...
bg`d xng gmz moxa gmog dbcxd gmz tovd gmog
... Electronegativities tend to either decrease down a group or remain about the same. Video clip on Electronegativity Example: Among the elements, gallium, bromine, and calcium, which has the highest electronegativity? Explain why in terms of periodic trends. The elements are all in the 4th period. Bro ...
... Electronegativities tend to either decrease down a group or remain about the same. Video clip on Electronegativity Example: Among the elements, gallium, bromine, and calcium, which has the highest electronegativity? Explain why in terms of periodic trends. The elements are all in the 4th period. Bro ...
Chapter 6 Notes
... Based on their electron configurations. The noble gases all have a full outer shell so they rarely take part in a reaction ...
... Based on their electron configurations. The noble gases all have a full outer shell so they rarely take part in a reaction ...
The Periodic Law - Mona Shores Blogs
... Atomic Radii – one-half the distance between the nuclei of identical atoms that are bonded together. o Atom size increases going down a group because electrons are being added to higher energy sublevels. (don’t worry about memorizing exceptions). o Atom size decreases going across a periods left to ...
... Atomic Radii – one-half the distance between the nuclei of identical atoms that are bonded together. o Atom size increases going down a group because electrons are being added to higher energy sublevels. (don’t worry about memorizing exceptions). o Atom size decreases going across a periods left to ...
Perioidicty Slide Show 2011
... Highly reactive (only have 1 outer electron to lose)-therefore not found in nature in elemental state; reacts with O2, to form oxide coating and H2O to form basic (alkali) solutions. Increase in reactivity down the group (Fr is most reactive metal). Softest of all metals (can be cut with a knife) an ...
... Highly reactive (only have 1 outer electron to lose)-therefore not found in nature in elemental state; reacts with O2, to form oxide coating and H2O to form basic (alkali) solutions. Increase in reactivity down the group (Fr is most reactive metal). Softest of all metals (can be cut with a knife) an ...
Ionization energy
... 2) The law did not work for very low or very high massed elements such as F, Cl, and Br. 3) As techniques improved for measuring atomic masses accurately, the law became obsolete. Dobereiner’s research made chemists look at groups of elements with similar chemical and physical properties. ...
... 2) The law did not work for very low or very high massed elements such as F, Cl, and Br. 3) As techniques improved for measuring atomic masses accurately, the law became obsolete. Dobereiner’s research made chemists look at groups of elements with similar chemical and physical properties. ...
Atoms
... A carbon atom always contains 6 protons. If it has an atomic mass of 13 how many neutrons are found in the nucleus? If a nitrogen atom has 8 neutrons in its nucleus what is the atomic number? ...
... A carbon atom always contains 6 protons. If it has an atomic mass of 13 how many neutrons are found in the nucleus? If a nitrogen atom has 8 neutrons in its nucleus what is the atomic number? ...
The Periodic Law
... – Due to the decrease of positive and negative charges from less protons and electrons and the attractive forces between them – The electrons are pulled closer to the nucleus from right to left & from bottom to bottom on the periodic table (which is why the A.R. decreases in these direction) ...
... – Due to the decrease of positive and negative charges from less protons and electrons and the attractive forces between them – The electrons are pulled closer to the nucleus from right to left & from bottom to bottom on the periodic table (which is why the A.R. decreases in these direction) ...
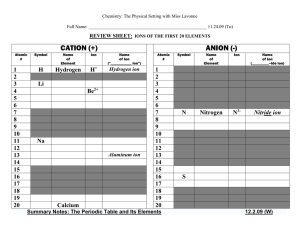
IONS OF THE FIRST 20 ELEMENTS
... The alkali metals, found in group 1 of the periodic table (formerly known as group IA), are very reactive metals that do not occur freely in nature. These metals have only one electron in their outer shell. Therefore, they are ready to lose that one electron in ionic bonding with other elements. As ...
... The alkali metals, found in group 1 of the periodic table (formerly known as group IA), are very reactive metals that do not occur freely in nature. These metals have only one electron in their outer shell. Therefore, they are ready to lose that one electron in ionic bonding with other elements. As ...
Atoms and the Periodic Table Mini quiz
... 1. The order of elements in the periodic table is based on a. the number of protons in the nucleus. c. the number of neutrons in the nucleus. b. the electric charge of the nucleus. d. atomic mass. 2. Atoms of elements that are in the same group have the same number of a. protons. c. valence electron ...
... 1. The order of elements in the periodic table is based on a. the number of protons in the nucleus. c. the number of neutrons in the nucleus. b. the electric charge of the nucleus. d. atomic mass. 2. Atoms of elements that are in the same group have the same number of a. protons. c. valence electron ...
periodic table - rosedalegrade9chemistry
... known elements in the early 1800’s. Could the elements be organized based on properties like colour, smell or taste? Not really, because the characteristics or properties were not unique. Early scientists found a property unique to each element, atomic mass. ...
... known elements in the early 1800’s. Could the elements be organized based on properties like colour, smell or taste? Not really, because the characteristics or properties were not unique. Early scientists found a property unique to each element, atomic mass. ...
Chapter 6
... One can predict physical properties such as the density, MP, atomic mass, and the atomic radius for an element in a group from examining trends for other elements in the group. Elements in the same group typically have similar chemical behavior and form similar compounds. For example, Na reacts with ...
... One can predict physical properties such as the density, MP, atomic mass, and the atomic radius for an element in a group from examining trends for other elements in the group. Elements in the same group typically have similar chemical behavior and form similar compounds. For example, Na reacts with ...
5SC19 Elements, Mixtures, and Compounds
... All atoms have a nucleus. The nucleus usually has neutrons and protons. Neutrons have no electrical charge and protons have a positive charge. An atom is identified by its number of protons, and that number is unique to that atom. For example, sodium has 11 protons, which means NO other atom has 11 ...
... All atoms have a nucleus. The nucleus usually has neutrons and protons. Neutrons have no electrical charge and protons have a positive charge. An atom is identified by its number of protons, and that number is unique to that atom. For example, sodium has 11 protons, which means NO other atom has 11 ...
Chapter 5 – The Periodic Law 5-1 History of the Periodic Table A
... 3. His first periodic table was published in ___________. a. He placed _________________, __ (atomic mass _________), after ____________________, ___ (atomic mass _________). It allowed him to place _______________________ in a group of elements with which it shares similar _________________________ ...
... 3. His first periodic table was published in ___________. a. He placed _________________, __ (atomic mass _________), after ____________________, ___ (atomic mass _________). It allowed him to place _______________________ in a group of elements with which it shares similar _________________________ ...
INTRO TO CHEMISTRY WEEK: SEPTEMBER 14 – 18, 2015
... atom has (see Table) o Noble gases don’t have any valence electrons because their outermost shells are totally full! That’s why they are inert o Atoms can do different things with their valence electrons: § Contribute them to a shared partnership with another atom à covalent bond § Totally donate ...
... atom has (see Table) o Noble gases don’t have any valence electrons because their outermost shells are totally full! That’s why they are inert o Atoms can do different things with their valence electrons: § Contribute them to a shared partnership with another atom à covalent bond § Totally donate ...
Periodic Table
... He moved cards to positions where they _______________________________________ This left ___________________ blank spaces. Mendeleev proposed that the blank spaces would be _____________________________ _________________________________________________________. He even predicted their ______________ ...
... He moved cards to positions where they _______________________________________ This left ___________________ blank spaces. Mendeleev proposed that the blank spaces would be _____________________________ _________________________________________________________. He even predicted their ______________ ...
Chapter 6 - The Periodic Table
... radius of an atom when it has become an ion. An ion is an atom or bonded group of atoms that has an overall positive or negative charge. An atom acquires a positive charge by losing electrons or negative charge by gaining electrons!! ...
... radius of an atom when it has become an ion. An ion is an atom or bonded group of atoms that has an overall positive or negative charge. An atom acquires a positive charge by losing electrons or negative charge by gaining electrons!! ...
Full Chapter - CPO Science
... gases or liquids in their pure form. Fluorine (F), chlorine (Cl), and bromine (Br) form salts when the bond with alkali metals. ...
... gases or liquids in their pure form. Fluorine (F), chlorine (Cl), and bromine (Br) form salts when the bond with alkali metals. ...
Name: ______ Date: _______________Class period: ______ Unit
... p) The amount of energy required to remove a second or third electron from an atom is always more than the amount of energy required to remove the first electron. q) For Potassium (K), we would expect a large increase in ionization energy to occur when the second (2nd) electron is removed. This is b ...
... p) The amount of energy required to remove a second or third electron from an atom is always more than the amount of energy required to remove the first electron. q) For Potassium (K), we would expect a large increase in ionization energy to occur when the second (2nd) electron is removed. This is b ...
Periodic Table - Marian High School
... gases or liquids in their pure form. Fluorine (F), chlorine (Cl), and bromine (Br) form salts when the bond with alkali metals. ...
... gases or liquids in their pure form. Fluorine (F), chlorine (Cl), and bromine (Br) form salts when the bond with alkali metals. ...
File - Lenora Henderson`s Flipped Chemistry Classroom
... Reading the Periodic Table Key Question What information can be displayed in a periodic table? The symbols and names of the elements, along with information about the structure of their atoms. Group 1A elements are called alkali metals Group 2A elements are called alkaline-earth metals Group ...
... Reading the Periodic Table Key Question What information can be displayed in a periodic table? The symbols and names of the elements, along with information about the structure of their atoms. Group 1A elements are called alkali metals Group 2A elements are called alkaline-earth metals Group ...
Noble gas

The noble gases make a group of chemical elements with similar properties. Under standard conditions, they are all odorless, colorless, monatomic gases with very low chemical reactivity. The six noble gases that occur naturally are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and the radioactive radon (Rn).For the first six periods of the periodic table, the noble gases are exactly the members of group 18 of the periodic table.It is possible that due to relativistic effects, the group 14 element flerovium exhibits some noble-gas-like properties, instead of the group 18 element ununoctium. Noble gases are typically highly unreactive except when under particular extreme conditions. The inertness of noble gases makes them very suitable in applications where reactions are not wanted. For example: argon is used in lightbulbs to prevent the hot tungsten filament from oxidizing; also, helium is breathed by deep-sea divers to prevent oxygen and nitrogen toxicity.The properties of the noble gases can be well explained by modern theories of atomic structure: their outer shell of valence electrons is considered to be ""full"", giving them little tendency to participate in chemical reactions, and it has been possible to prepare only a few hundred noble gas compounds. The melting and boiling points for a given noble gas are close together, differing by less than 10 °C (18 °F); that is, they are liquids over only a small temperature range.Neon, argon, krypton, and xenon are obtained from air in an air separation unit using the methods of liquefaction of gases and fractional distillation. Helium is sourced from natural gas fields which have high concentrations of helium in the natural gas, using cryogenic gas separation techniques, and radon is usually isolated from the radioactive decay of dissolved radium, thorium, or uranium compounds (since those compounds give off alpha particles). Noble gases have several important applications in industries such as lighting, welding, and space exploration. A helium-oxygen breathing gas is often used by deep-sea divers at depths of seawater over 55 m (180 ft) to keep the diver from experiencing oxygen toxemia, the lethal effect of high-pressure oxygen, and nitrogen narcosis, the distracting narcotic effect of the nitrogen in air beyond this partial-pressure threshold. After the risks caused by the flammability of hydrogen became apparent, it was replaced with helium in blimps and balloons.