Document
... A quantity of 1.00 × 102 mL of 0.500 M HCl was mixed with 1.00 × 102 mL of 0.500 M NaOH in a constant-pressure calorimeter of negligible heat capacity. The initial temperature of the HCl and NaOH solutions was the same, 22.50°C, and the final temperature of the mixed solution was 25.86°C. Calculate ...
... A quantity of 1.00 × 102 mL of 0.500 M HCl was mixed with 1.00 × 102 mL of 0.500 M NaOH in a constant-pressure calorimeter of negligible heat capacity. The initial temperature of the HCl and NaOH solutions was the same, 22.50°C, and the final temperature of the mixed solution was 25.86°C. Calculate ...
Practice Writing AP Questions
... 11. A piece of lithium metal is dropped into a container of nitrogen gas. a. If the container was sealed quickly, what would you expect to happen to the pressure inside the container as the reaction proceeds? 12. Powdered magnesium oxide is added to a container of carbon dioxide gas. a. State the ox ...
... 11. A piece of lithium metal is dropped into a container of nitrogen gas. a. If the container was sealed quickly, what would you expect to happen to the pressure inside the container as the reaction proceeds? 12. Powdered magnesium oxide is added to a container of carbon dioxide gas. a. State the ox ...
Class Notes
... The law of conservation of mass (aka: the law of conservation of matter) says that during a chemical reaction or a physical change mass is conserved; mass is neither created nor destroyed. This implies that the atoms that were there in the reactants (before the chemical change) must be there in the ...
... The law of conservation of mass (aka: the law of conservation of matter) says that during a chemical reaction or a physical change mass is conserved; mass is neither created nor destroyed. This implies that the atoms that were there in the reactants (before the chemical change) must be there in the ...
Balancing and Predicting Chemical Reactions:
... 2. Aqueous nitric acid and calcium hydroxide solutions react to form water and aqueous calcium nitrate Word equation: nitric acid(aq) + calcium hydroxide(aq) water(l) + calcium nitrate(aq) Skeleton formula equation: HNO3(aq) + Ca(OH)2(aq) H2O(l) + Ca(NO3)2(aq) ...
... 2. Aqueous nitric acid and calcium hydroxide solutions react to form water and aqueous calcium nitrate Word equation: nitric acid(aq) + calcium hydroxide(aq) water(l) + calcium nitrate(aq) Skeleton formula equation: HNO3(aq) + Ca(OH)2(aq) H2O(l) + Ca(NO3)2(aq) ...
C:\My Documents\My Documents\Teaching\chem130\hunt
... two basic chemistry topics: (1) chemical reactions and stoichiometry (mole relationships, chemical equations and chemical reaction, sequential reactions, limiting reactants, net ionic equations, gravimetric analysis and volumetric analysis) and (2) thermochemistry (thermochemical equations, standard ...
... two basic chemistry topics: (1) chemical reactions and stoichiometry (mole relationships, chemical equations and chemical reaction, sequential reactions, limiting reactants, net ionic equations, gravimetric analysis and volumetric analysis) and (2) thermochemistry (thermochemical equations, standard ...
General Chemistry Unit 11
... In a synthesis reaction two or more simple substances combine to form a more complex substance. Two or more reactants yielding one product is another way to identify a synthesis reaction. For example, simple hydrogen gas combined with simple oxygen gas can produce a more complex substance----water! ...
... In a synthesis reaction two or more simple substances combine to form a more complex substance. Two or more reactants yielding one product is another way to identify a synthesis reaction. For example, simple hydrogen gas combined with simple oxygen gas can produce a more complex substance----water! ...
p Block Elements General Configuration: ns2 np1
... that they cannot have effective overlapping. P, As and Sb form P-P, As-As and Sb-Sb single bonds whereas Bi forms metallic bonds. However, N-N single bond is weaker than P-P single bond, because of high inter electronic repulsion of non-bonding electrons owing to small bond length. Catenation tenden ...
... that they cannot have effective overlapping. P, As and Sb form P-P, As-As and Sb-Sb single bonds whereas Bi forms metallic bonds. However, N-N single bond is weaker than P-P single bond, because of high inter electronic repulsion of non-bonding electrons owing to small bond length. Catenation tenden ...
The Advanced Placement Examination in Chemistry Part I – Multiple
... (d) Compound Z contains carbon, hydrogen, and element Q. When 1.00 gram of compound Z is oxidized and all of the carbon and hydrogen are converted to oxides, 1.37 grams of CO2 and 0.281 gram of water are produced. Determine the most probable molecular formula of compound Z. ...
... (d) Compound Z contains carbon, hydrogen, and element Q. When 1.00 gram of compound Z is oxidized and all of the carbon and hydrogen are converted to oxides, 1.37 grams of CO2 and 0.281 gram of water are produced. Determine the most probable molecular formula of compound Z. ...
2012 Chem 13 News Exam
... Starting from reactants only, and before equilibrium is established, the rate of the forward reaction continually increases. ...
... Starting from reactants only, and before equilibrium is established, the rate of the forward reaction continually increases. ...
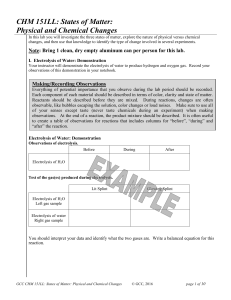
CHM 151LL: States of Matter: Physical and Chemical Changes
... Chemists in industry and research are interested in the changes substances undergo in order to provide better products to the marketplace and to discover new potentially useful substances. Chemical changes do change the composition of the atoms or molecules in the substance. They result in new subst ...
... Chemists in industry and research are interested in the changes substances undergo in order to provide better products to the marketplace and to discover new potentially useful substances. Chemical changes do change the composition of the atoms or molecules in the substance. They result in new subst ...
Problem 1: “A brief history” of life in the universe
... chemical reactions involving atoms and molecules. It is only natural then to ask where atoms came from. According to a widely accepted model, the universe began about 15 billion years ago in a big bang and has been expanding ever since. The history of the universe as a whole can be viewed in terms o ...
... chemical reactions involving atoms and molecules. It is only natural then to ask where atoms came from. According to a widely accepted model, the universe began about 15 billion years ago in a big bang and has been expanding ever since. The history of the universe as a whole can be viewed in terms o ...
Problem 1: “A brief history” of life in the universe
... The chemical composition of the atmosphere of a planet depends on the temperature of the planet’s atmosphere (which in turn depends on the distance from the sun, internal temperature, etc.), tectonic activity, and the existence of life. As the sun generated heat, light, and solar wind through nuclea ...
... The chemical composition of the atmosphere of a planet depends on the temperature of the planet’s atmosphere (which in turn depends on the distance from the sun, internal temperature, etc.), tectonic activity, and the existence of life. As the sun generated heat, light, and solar wind through nuclea ...
Problem 1: A brief history of life in the universe
... The chemical composition of the atmosphere of a planet depends on the temperature of the planet’s atmosphere (which in turn depends on the distance from the sun, internal temperature, etc.), tectonic activity, and the existence of life. As the sun generated heat, light, and solar wind through nuclea ...
... The chemical composition of the atmosphere of a planet depends on the temperature of the planet’s atmosphere (which in turn depends on the distance from the sun, internal temperature, etc.), tectonic activity, and the existence of life. As the sun generated heat, light, and solar wind through nuclea ...
Classifying Chemical Reactions by What Atoms Do
... (More precisely, the H+ from the acid molecule is donated to a water molecule to form hydronium ion, H3O+) Bases dissociate in water to form OH- ions. (Bases, such as NH3, that do not contain OH- ions, produce OH- by pulling H+ off water molecules.) In the reaction of an acid with a base, the H+ fro ...
... (More precisely, the H+ from the acid molecule is donated to a water molecule to form hydronium ion, H3O+) Bases dissociate in water to form OH- ions. (Bases, such as NH3, that do not contain OH- ions, produce OH- by pulling H+ off water molecules.) In the reaction of an acid with a base, the H+ fro ...
Chemistry Chapter 9.1 Making Predictions About Solubility
... PRECIPITATE REACTION – a double displacement reaction that results in the formation of an insoluble substance - Use the general solubility guidelines to predict if a compound is soluble or insoluble - The element with the higher class will determine if the compound is soluble or insoluble DOUBLE DIS ...
... PRECIPITATE REACTION – a double displacement reaction that results in the formation of an insoluble substance - Use the general solubility guidelines to predict if a compound is soluble or insoluble - The element with the higher class will determine if the compound is soluble or insoluble DOUBLE DIS ...
Reaction Stoichiometry
... When copper metal is added to silver nitrate in solution, silver metal and copper (II) nitrate are produced. What mass of silver is produced from 100.0g of Cu? What mass of aluminum is produced by the decomposition of 5.0 kg of Al2O3? ...
... When copper metal is added to silver nitrate in solution, silver metal and copper (II) nitrate are produced. What mass of silver is produced from 100.0g of Cu? What mass of aluminum is produced by the decomposition of 5.0 kg of Al2O3? ...
Keq Assignment
... 5. For the reaction: carbon monoxide burns in oxygen to produce carbon dioxide You are given the following equilibrium conditions: [O2] = 1.30 × 10-3M [CO2] = 2.50 × 10-4M Keq = 3.60 × 10-3M Write the balanced equilibrium equation and calculate [CO] ...
... 5. For the reaction: carbon monoxide burns in oxygen to produce carbon dioxide You are given the following equilibrium conditions: [O2] = 1.30 × 10-3M [CO2] = 2.50 × 10-4M Keq = 3.60 × 10-3M Write the balanced equilibrium equation and calculate [CO] ...
Water splitting

Water splitting is the general term for a chemical reaction in which water is separated into oxygen and hydrogen. Efficient and economical water splitting would be a key technology component of a hydrogen economy. Various techniques for water splitting have been issued in water splitting patents in the United States. In photosynthesis, water splitting donates electrons to power the electron transport chain in photosystem II.