Rxn Types
... Decomposition of a binary compound When the right energy is applied to a binary compound it will break apart into its respective elements. ...
... Decomposition of a binary compound When the right energy is applied to a binary compound it will break apart into its respective elements. ...
Higher Chemistry - Mobile Resource
... molecules are moving slowly they will have a ‘soft’ collision. They will simply bounce off each other without reacting. If 2 colliding molecules are moving fast enough they will have a ‘hard’ collision in which bonds will break and allow the reaction to happen. Clearly there must be a minimum speed ...
... molecules are moving slowly they will have a ‘soft’ collision. They will simply bounce off each other without reacting. If 2 colliding molecules are moving fast enough they will have a ‘hard’ collision in which bonds will break and allow the reaction to happen. Clearly there must be a minimum speed ...
stoichiometry
... 25) How many moles of hydrogen can be produced from 8.40 g of aluminum and excess sodium hydroxide? How many grams? 2Al + 6NaOH 2Na3AlO3 + 3H2 ...
... 25) How many moles of hydrogen can be produced from 8.40 g of aluminum and excess sodium hydroxide? How many grams? 2Al + 6NaOH 2Na3AlO3 + 3H2 ...
New Advances in Catalytic Systems for Conversion of CH4 and CO2
... for natural gas; (2) liquid phase carbonylation technology of CH3 OH and related homogeneous catalysis systems; (3) indirect conversion of C1 raw materials to C2 H4 , C2 H5 OH, CH3 COOH and liquid fuels via syngas. As a whole, the basis of the above achievements is the syngas manufacturing technolog ...
... for natural gas; (2) liquid phase carbonylation technology of CH3 OH and related homogeneous catalysis systems; (3) indirect conversion of C1 raw materials to C2 H4 , C2 H5 OH, CH3 COOH and liquid fuels via syngas. As a whole, the basis of the above achievements is the syngas manufacturing technolog ...
CHEM_01A_ExptD_Copper_Cycle_F14
... All steps in this experiment will be performed under the fume hood. Your instructor will turn on the fume hoods before you begin the experiment. Be sure to wear your safety goggles and lab jacket ...
... All steps in this experiment will be performed under the fume hood. Your instructor will turn on the fume hoods before you begin the experiment. Be sure to wear your safety goggles and lab jacket ...
Unit - 7.pmd
... Ammonia is a colourless gas with a pungent odour. Its freezing and boiling points are 198.4 and 239.7 K respectively. In the solid and liquid states, it is associated through hydrogen bonds as in the case of water and that accounts for its higher melting and boiling points than expected on the basis ...
... Ammonia is a colourless gas with a pungent odour. Its freezing and boiling points are 198.4 and 239.7 K respectively. In the solid and liquid states, it is associated through hydrogen bonds as in the case of water and that accounts for its higher melting and boiling points than expected on the basis ...
Chemical Equations and Stoichiometry
... Because of propane’s simple chemical structure, it usually burns cleanly. Unlike gasoline, in a wellventilated area, it will not produce much soot or carbon monoxide. It does however produce the greenhouse gas carbon dioxide, which has been accumulating in the atmosphere since the Industrial Revolut ...
... Because of propane’s simple chemical structure, it usually burns cleanly. Unlike gasoline, in a wellventilated area, it will not produce much soot or carbon monoxide. It does however produce the greenhouse gas carbon dioxide, which has been accumulating in the atmosphere since the Industrial Revolut ...
Exam 2
... A. a measure of the energy absorbed when four 1H are converted to one 4He. B. due to the loss of two electrons when four 1H are converted to one 4He. C. a measure of the energy released when four 1H are converted to one 4He. D. equal to the mass of the two positrons produced when four 1H are convert ...
... A. a measure of the energy absorbed when four 1H are converted to one 4He. B. due to the loss of two electrons when four 1H are converted to one 4He. C. a measure of the energy released when four 1H are converted to one 4He. D. equal to the mass of the two positrons produced when four 1H are convert ...
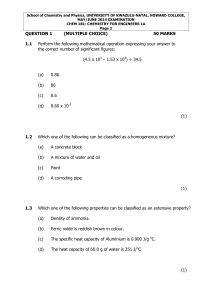
School of Chemistry
... School of Chemistry and Physics, UNIVERSITY OF KWAZULU-NATAL, HOWARD COLLEGE, MAY/JUNE 2014 EXAMINATION CHEM 181: CHEMISTRY FOR ENGINEERS 1A Page 10 ...
... School of Chemistry and Physics, UNIVERSITY OF KWAZULU-NATAL, HOWARD COLLEGE, MAY/JUNE 2014 EXAMINATION CHEM 181: CHEMISTRY FOR ENGINEERS 1A Page 10 ...
12 U Chem Review
... a compound with a structure based on benzene (a ring of six carbons) consider the benzene ring to be the parent molecule alkyl groups are named to give the lowest combination of numbers, with no particular starting carbon (as it is a ring) when it is easier to consider the benzene ring as an alk ...
... a compound with a structure based on benzene (a ring of six carbons) consider the benzene ring to be the parent molecule alkyl groups are named to give the lowest combination of numbers, with no particular starting carbon (as it is a ring) when it is easier to consider the benzene ring as an alk ...
sch4ureview
... a compound with a structure based on benzene (a ring of six carbons) consider the benzene ring to be the parent molecule alkyl groups are named to give the lowest combination of numbers, with no particular starting carbon (as it is a ring) when it is easier to consider the benzene ring as an alk ...
... a compound with a structure based on benzene (a ring of six carbons) consider the benzene ring to be the parent molecule alkyl groups are named to give the lowest combination of numbers, with no particular starting carbon (as it is a ring) when it is easier to consider the benzene ring as an alk ...
Chemistry 12 - Correspondence Studies
... The amount of energy involved in a chemical reaction can be measured using an instrument called a calorimeter. A simple laboratory calorimeter is shown on page 357 of the text. The polystyrene cup acts as an insulator to reduce heat flow to the surroundings. The chemical reaction takes place in the ...
... The amount of energy involved in a chemical reaction can be measured using an instrument called a calorimeter. A simple laboratory calorimeter is shown on page 357 of the text. The polystyrene cup acts as an insulator to reduce heat flow to the surroundings. The chemical reaction takes place in the ...
AP Chemistry
... in the bond angles in the series of molecules CH4, NH3, and H2O is best accounted for by the (A) increasing strength of the bonds (B) decreasing size of the central atom (C) increasing electronegativity of the central atom (D) increasing number of unshared pairs of electrons ...
... in the bond angles in the series of molecules CH4, NH3, and H2O is best accounted for by the (A) increasing strength of the bonds (B) decreasing size of the central atom (C) increasing electronegativity of the central atom (D) increasing number of unshared pairs of electrons ...
Types of Aqueous Reactions
... Another type of aqueous chemical reaction is an acid/base reaction – the reaction between an acid and a base. There are different types of acids and bases. We rely on the Bronsted-Lowry definition: ...
... Another type of aqueous chemical reaction is an acid/base reaction – the reaction between an acid and a base. There are different types of acids and bases. We rely on the Bronsted-Lowry definition: ...
physical setting chemistry
... Part C Answer all questions in this part. Directions (68–85): Record your answers in the spaces provided in your answer booklet. Some questions may require the use of the Reference Tables for Physical Setting/Chemistry. Base your answers to questions 68 through 70 on the information and table below ...
... Part C Answer all questions in this part. Directions (68–85): Record your answers in the spaces provided in your answer booklet. Some questions may require the use of the Reference Tables for Physical Setting/Chemistry. Base your answers to questions 68 through 70 on the information and table below ...
BONUS: Which line in the above graph represents G for the reaction
... The Arrhenius equation, k = Ae-E/RT expresses the relationship between the reaction rate constant, k, and the energy of activation, E. The probability that colliding molecules will react (A) ...
... The Arrhenius equation, k = Ae-E/RT expresses the relationship between the reaction rate constant, k, and the energy of activation, E. The probability that colliding molecules will react (A) ...
Chemistry II Exams and Keys Corrected 2016 Season
... 13. What is the empirical formula of aspartame, if aspartame is an artificial sweetener that is found to be 57.14% carbon, 6.16% hydrogen, 9.52% nitrogen, and 27.18% oxygen? A. C5H10NO ...
... 13. What is the empirical formula of aspartame, if aspartame is an artificial sweetener that is found to be 57.14% carbon, 6.16% hydrogen, 9.52% nitrogen, and 27.18% oxygen? A. C5H10NO ...
oxidation–reduction reaction
... • A reaction in which electrons are transferred from one atom to another is called an oxidation–reduction reaction. • Also called redox reactions ...
... • A reaction in which electrons are transferred from one atom to another is called an oxidation–reduction reaction. • Also called redox reactions ...
Water splitting

Water splitting is the general term for a chemical reaction in which water is separated into oxygen and hydrogen. Efficient and economical water splitting would be a key technology component of a hydrogen economy. Various techniques for water splitting have been issued in water splitting patents in the United States. In photosynthesis, water splitting donates electrons to power the electron transport chain in photosystem II.