Structure and Properties of Matter
... We have just seen, the first chemist to use the name ‘atom’ was John Dalton. Dalton used the word ‘atom’ to mean the smallest particle of an element. He then went on explaining how atoms could react together to form molecules; which he called ‘compound atoms’. Today we know what a molecule is. A mol ...
... We have just seen, the first chemist to use the name ‘atom’ was John Dalton. Dalton used the word ‘atom’ to mean the smallest particle of an element. He then went on explaining how atoms could react together to form molecules; which he called ‘compound atoms’. Today we know what a molecule is. A mol ...
Unit 1 4 Energy, Chemical Reactions and Physical Changes
... Thus, heat is the sibling of work, and heat is often referred to as a form of energy (but “heat” per se, is not on the electrochemical spectrum [infrared energy is … but not, heat] Heat really is equivalent to the amount of energy that is transferred between the reacting chemicals and surroundings. ...
... Thus, heat is the sibling of work, and heat is often referred to as a form of energy (but “heat” per se, is not on the electrochemical spectrum [infrared energy is … but not, heat] Heat really is equivalent to the amount of energy that is transferred between the reacting chemicals and surroundings. ...
Elements – (Metals)
... Low melting point, low boiling point (Cs M.P. = 29 oC) Soft (can cut with steel knife) Have one electron beyond noble gas so form +1 ions easy to get off first electron, difficult to get off 2nd electron Good reducing agents because they will undergo oxidation Li Li+ + e- occurs readily Reaction e ...
... Low melting point, low boiling point (Cs M.P. = 29 oC) Soft (can cut with steel knife) Have one electron beyond noble gas so form +1 ions easy to get off first electron, difficult to get off 2nd electron Good reducing agents because they will undergo oxidation Li Li+ + e- occurs readily Reaction e ...
synthesis-structure relationship in the aqueous ethylene glycol
... Fe(NO3)3·9H2O, EG (99%) and 1.5 M HNO3, all from “Reactivul” Bucharest, were used. The subsequent purification step applied to the coordination compound assures the removal of most reagent impurities and reaction byproducts, so the desired product is obtained in high purity. The water content was de ...
... Fe(NO3)3·9H2O, EG (99%) and 1.5 M HNO3, all from “Reactivul” Bucharest, were used. The subsequent purification step applied to the coordination compound assures the removal of most reagent impurities and reaction byproducts, so the desired product is obtained in high purity. The water content was de ...
REDOX EQUILIBRIA SL - chemistryatdulwich
... (b) 2NaCl (aq) + F2 (aq) 2NaF (aq) + CI2 (aq) (c) CuSO4 (aq) + Zn (s) ZnSO4 (aq) + Cu (s) (d) Cu(s) + 2AgNO3 (aq) → Cu(NO3)2 (aq) + 2Ag(s) (e) CuO (s) + H2 (g) Cu (s) + H2O (l) (f) 2Fe2+(aq) + I2(aq) 2Fe3+(aq) + 2I-(aq) (g) Sn2+ (aq) + 2Fe3+ (aq) Sn4+ (aq) + 2Fe2+ (aq) (h) 3I2(aq) + 3OH - ...
... (b) 2NaCl (aq) + F2 (aq) 2NaF (aq) + CI2 (aq) (c) CuSO4 (aq) + Zn (s) ZnSO4 (aq) + Cu (s) (d) Cu(s) + 2AgNO3 (aq) → Cu(NO3)2 (aq) + 2Ag(s) (e) CuO (s) + H2 (g) Cu (s) + H2O (l) (f) 2Fe2+(aq) + I2(aq) 2Fe3+(aq) + 2I-(aq) (g) Sn2+ (aq) + 2Fe3+ (aq) Sn4+ (aq) + 2Fe2+ (aq) (h) 3I2(aq) + 3OH - ...
UNIT 5 - H-W Science Website
... the form of heat, but in some processes it may take the form of mainly light, or a mixture of forms including some mechanical energy such as sound. Whatever the case, the conclusion we could draw from this aspect of chemical change is that energy is somehow stored in the chemicals and during a trans ...
... the form of heat, but in some processes it may take the form of mainly light, or a mixture of forms including some mechanical energy such as sound. Whatever the case, the conclusion we could draw from this aspect of chemical change is that energy is somehow stored in the chemicals and during a trans ...
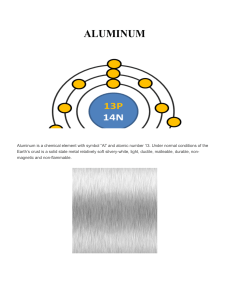
ALUMINUM
... Aluminum is highly reactive and it is very rare to find natively on the crust, so it is a very versatile, which can constitute about 300 alloys and a lot of variants. Despite this, aluminum does not ignite instantly, so it has many applications in handling flammable or explosive materials. Aluminum ...
... Aluminum is highly reactive and it is very rare to find natively on the crust, so it is a very versatile, which can constitute about 300 alloys and a lot of variants. Despite this, aluminum does not ignite instantly, so it has many applications in handling flammable or explosive materials. Aluminum ...
Chapter 5 Thermochemistry
... overall reaction will be equal to the sum of the enthalpy changes for the individual steps.” ...
... overall reaction will be equal to the sum of the enthalpy changes for the individual steps.” ...
Combining the Benefits of Homogeneous and Heterogeneous
... such as acetonitrile, dioxane, and THF that can be used for homogeneously catalyzed reactions. Modest pressures of a soluble gas, generally CO2, achieve facile post-reaction heterogeneous separation of products from the catalyst. Examples shown here are rhodiumcatalyzed hydroformylation of 1-octene ...
... such as acetonitrile, dioxane, and THF that can be used for homogeneously catalyzed reactions. Modest pressures of a soluble gas, generally CO2, achieve facile post-reaction heterogeneous separation of products from the catalyst. Examples shown here are rhodiumcatalyzed hydroformylation of 1-octene ...
Name: Period:______ Let`s make some sandwiches! Introduction: If
... Name:______________________ Period:_________ Let’s make some sandwiches! Introduction: If a sandwich shop runs out of bread, the shop closes down. No more sandwiches can be fully made without ordering more bread from a bakery. A similar thing happens in a chemical reaction. If there are fixed amount ...
... Name:______________________ Period:_________ Let’s make some sandwiches! Introduction: If a sandwich shop runs out of bread, the shop closes down. No more sandwiches can be fully made without ordering more bread from a bakery. A similar thing happens in a chemical reaction. If there are fixed amount ...
Chapter 5 Thermochemistry
... overall reaction will be equal to the sum of the enthalpy changes for the individual steps.” ...
... overall reaction will be equal to the sum of the enthalpy changes for the individual steps.” ...
21:3 Classifying Chemical Reactions
... supplement. Yeasts are found in the soil, in water, on the surface of plants, and on the skin of humans and other animals. Like other fungi, yeasts obtain food from the organic matter around them; they secrete enzymes that break down the organic matter into nutrients they can absorb. As yeast live a ...
... supplement. Yeasts are found in the soil, in water, on the surface of plants, and on the skin of humans and other animals. Like other fungi, yeasts obtain food from the organic matter around them; they secrete enzymes that break down the organic matter into nutrients they can absorb. As yeast live a ...
chapter 13 - Humble ISD
... If the Keq < 1, then Reactants are favored If the Keq = 1, then Products and Reactants are equal If the Keq > 1 then Products are favored Since the Keq is 19.5 and 19.5 is greater then 1, more Products will be present then ...
... If the Keq < 1, then Reactants are favored If the Keq = 1, then Products and Reactants are equal If the Keq > 1 then Products are favored Since the Keq is 19.5 and 19.5 is greater then 1, more Products will be present then ...
Term 111, Final Exam (All correct choices are A): 1. What is the
... A) The solubility of solids in water always increases with increasing temperature B) The solubility of solids in water always decreases with increasing temperature C) The solubility of a gas in water increases with increasing temperature D) The solubility of NH3 gas in water is well described by Hen ...
... A) The solubility of solids in water always increases with increasing temperature B) The solubility of solids in water always decreases with increasing temperature C) The solubility of a gas in water increases with increasing temperature D) The solubility of NH3 gas in water is well described by Hen ...
Water splitting

Water splitting is the general term for a chemical reaction in which water is separated into oxygen and hydrogen. Efficient and economical water splitting would be a key technology component of a hydrogen economy. Various techniques for water splitting have been issued in water splitting patents in the United States. In photosynthesis, water splitting donates electrons to power the electron transport chain in photosystem II.