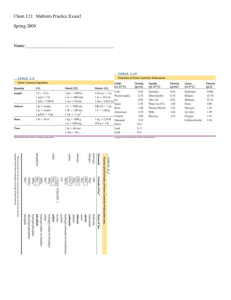
2009-10 Chemistry 1st Semester Final Exam Topics and Review
... d. Hydrogen gas reacts with nitrogen gas to form ammonia. Write and balance a chemical equation for this reaction. e. One of the problems with space travel is the building up of carbon dioxide produced by the astronauts. The typical procedure is to react the carbon dioxide with lithium hydroxide to ...
... d. Hydrogen gas reacts with nitrogen gas to form ammonia. Write and balance a chemical equation for this reaction. e. One of the problems with space travel is the building up of carbon dioxide produced by the astronauts. The typical procedure is to react the carbon dioxide with lithium hydroxide to ...
Name: Date: AP Chemistry/Chemistry 145 Summer Assignment
... 16. Limestone, coral, and seashells are composed primarily of solid calcium carbonate. The test for the identification of a carbonate is to use a few drops of aqueous hydrochloric acid. Solid ...
... 16. Limestone, coral, and seashells are composed primarily of solid calcium carbonate. The test for the identification of a carbonate is to use a few drops of aqueous hydrochloric acid. Solid ...
Unit 3 Test - hrsbstaff.ednet.ns.ca
... The following document is a teaching resource available at www.MorrowGalpern.ca ...
... The following document is a teaching resource available at www.MorrowGalpern.ca ...
Sustainable Hydrogen and Electrical Energy Storage 5 Interested in
... pre-cooling is necessary if expansion without a piston is used (e.g. through a valve into a fixed volume). Why heating above inversion T? At high T there are more collisions between the molecules. During collision → at higher potential energy → lower ...
... pre-cooling is necessary if expansion without a piston is used (e.g. through a valve into a fixed volume). Why heating above inversion T? At high T there are more collisions between the molecules. During collision → at higher potential energy → lower ...
Solution
... favored. This is consistent with the negative free energy of part (c). e) The pressure of oxygen is 5 atm and the pressure of hydrogen is 10 atm at 25°C. In which direction will the reaction shift in order to regain equilibrium. Show all work and explain your reasoning. We have K calculated above an ...
... favored. This is consistent with the negative free energy of part (c). e) The pressure of oxygen is 5 atm and the pressure of hydrogen is 10 atm at 25°C. In which direction will the reaction shift in order to regain equilibrium. Show all work and explain your reasoning. We have K calculated above an ...
The Living Planet
... photosynthetic organisms trap convert light energy into chemical bonds…typically by binding a phosphate (PO4) to adenosine diphosphate (ADP) to make ATP (adenosine triphosphate). To get the electrons that carry this radiant energy, the organisms split water molecules… the freed oxygen atoms from 2 w ...
... photosynthetic organisms trap convert light energy into chemical bonds…typically by binding a phosphate (PO4) to adenosine diphosphate (ADP) to make ATP (adenosine triphosphate). To get the electrons that carry this radiant energy, the organisms split water molecules… the freed oxygen atoms from 2 w ...
Chemical Reactions - Mr. Brown`s Science Town
... Evidence of a Chemical Reaction Production of a gas/bubbles A solid (precipitate) forms Color changes Energy is released (light, flames, ...
... Evidence of a Chemical Reaction Production of a gas/bubbles A solid (precipitate) forms Color changes Energy is released (light, flames, ...
Unit 1
... Unsaturated At least one double bond between 1 pair of carbon atoms. Liquid at room temperature Most vegetable fats ...
... Unsaturated At least one double bond between 1 pair of carbon atoms. Liquid at room temperature Most vegetable fats ...
2 - CronScience
... b) Some metallic oxides react with water to produce a base: CaO + H2O Ca(OH)2 ...
... b) Some metallic oxides react with water to produce a base: CaO + H2O Ca(OH)2 ...
Unit 3 Practice Test
... 7. Identify the INCORRECT statement below: A. Non-metals generally have the higher electronegativities and tend to attract electrons to themselves in a chemical bond. B. Elements with high ionization energies tend to have small atomic radii. C. Elements with high electronegativities generally form i ...
... 7. Identify the INCORRECT statement below: A. Non-metals generally have the higher electronegativities and tend to attract electrons to themselves in a chemical bond. B. Elements with high ionization energies tend to have small atomic radii. C. Elements with high electronegativities generally form i ...
Chemistry- CST Review
... 8. If a sample of gas occupies 6.55 L at 300 °C, what will be its volume at 25 °C if the pressure does not change? 9. A gas at 790 mm Hg and 25 °C occupies a container with an initial volume of 1.20 L. By changing the volume, the pressure of the gas increases to 1500 mm Hg as the temperature is rais ...
... 8. If a sample of gas occupies 6.55 L at 300 °C, what will be its volume at 25 °C if the pressure does not change? 9. A gas at 790 mm Hg and 25 °C occupies a container with an initial volume of 1.20 L. By changing the volume, the pressure of the gas increases to 1500 mm Hg as the temperature is rais ...
7. Diagrams of all experiments
... Qualitative food tests for starch, reducing sugar, protein and fat. Investigate the conversion of chemical energy in food to heat energy. Investigate the action of amylase on starch; identify the substrate, product and enzyme. Carry out qualitative tests to compare the carbon dioxide levels of inhal ...
... Qualitative food tests for starch, reducing sugar, protein and fat. Investigate the conversion of chemical energy in food to heat energy. Investigate the action of amylase on starch; identify the substrate, product and enzyme. Carry out qualitative tests to compare the carbon dioxide levels of inhal ...
Matter 1. ______ is anything that has ______ and takes up ______
... b. _________________- a description of the way a substance reacts to become a new substance. For example: - water reacts vigorously with the metal sodium to produce hydrogen. - by means of electricity, water decomposes to form hydrogen and oxygen. c. properties can also be classified as extensive or ...
... b. _________________- a description of the way a substance reacts to become a new substance. For example: - water reacts vigorously with the metal sodium to produce hydrogen. - by means of electricity, water decomposes to form hydrogen and oxygen. c. properties can also be classified as extensive or ...
Water splitting

Water splitting is the general term for a chemical reaction in which water is separated into oxygen and hydrogen. Efficient and economical water splitting would be a key technology component of a hydrogen economy. Various techniques for water splitting have been issued in water splitting patents in the United States. In photosynthesis, water splitting donates electrons to power the electron transport chain in photosystem II.