2nd Semester Exam Review
... Assumptions of the Kinetic Molecular Theory • Gasses consist of small particles that take up little volume relative to the volume of empty space around them – Gas molecules are very far apart and therefore don’t experience attractive or repulsive forces. ...
... Assumptions of the Kinetic Molecular Theory • Gasses consist of small particles that take up little volume relative to the volume of empty space around them – Gas molecules are very far apart and therefore don’t experience attractive or repulsive forces. ...
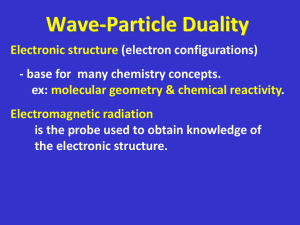
Wave Particle Duality Power Point NOTES
... De Broglie’s Equation • Broglie’s stated that an object in motion behaves as both particles & waves, just as light does. • Electrons are WAVES in FIXED LOCATIONS which means they are QUANTIZED (quantity) wavelengths. ...
... De Broglie’s Equation • Broglie’s stated that an object in motion behaves as both particles & waves, just as light does. • Electrons are WAVES in FIXED LOCATIONS which means they are QUANTIZED (quantity) wavelengths. ...
States of Matter
... Variables are pressure (y) and temperature (x) Triple point: temperature and pressure where solid, liquid and gas can exist in equilibrium Critical temperature: temperature above which a substance cannot be liquified Critical pressure: pressure necessary to liquefy a substance at the critical temper ...
... Variables are pressure (y) and temperature (x) Triple point: temperature and pressure where solid, liquid and gas can exist in equilibrium Critical temperature: temperature above which a substance cannot be liquified Critical pressure: pressure necessary to liquefy a substance at the critical temper ...
Nome Completo: Ana Valéria Colnaghi Simionato
... Pharmaceutical residues have been detected in the environment as a consequence of their widespread usage in hospitals, industries, agriculture, livestock and household. However, these drug residues usually occur at low concentration (ng L1 ) in the environment. Therefore, mass spectrometric (MS) det ...
... Pharmaceutical residues have been detected in the environment as a consequence of their widespread usage in hospitals, industries, agriculture, livestock and household. However, these drug residues usually occur at low concentration (ng L1 ) in the environment. Therefore, mass spectrometric (MS) det ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... corresponding eigen value. 2. What is a linear operator? Explain it with a suitable example. 3. What is zero-point energy? What is its value for a particle in a 1-D box? 4. Write the time-dependent Schroedinger wave equation for a single particle in 1-D in stationary state. 5. Draw the radial probab ...
... corresponding eigen value. 2. What is a linear operator? Explain it with a suitable example. 3. What is zero-point energy? What is its value for a particle in a 1-D box? 4. Write the time-dependent Schroedinger wave equation for a single particle in 1-D in stationary state. 5. Draw the radial probab ...
FeCo magnetic nanoneedles obtained by Co-coating
... 10 and 20% of Co, using the same procedure as that used with haematite and then subjected again to the first reduction step (see figure 1). In this way, single-phase magnetite particles with 18 and 27% of Co were obtained, according to the chemical analyses, without segregation of other phases as il ...
... 10 and 20% of Co, using the same procedure as that used with haematite and then subjected again to the first reduction step (see figure 1). In this way, single-phase magnetite particles with 18 and 27% of Co were obtained, according to the chemical analyses, without segregation of other phases as il ...
Science 9 Unit 2
... Heterogeneous Mixtures Heterogeneous mixtures is a mechanical mixture where you can see the parts There are three types of heterogeneous mixtures based on the size of the particles. Ordinary mechanical mixtures have parts big enough to see and they stay mixed. E.g. granite Suspensions are large mix ...
... Heterogeneous Mixtures Heterogeneous mixtures is a mechanical mixture where you can see the parts There are three types of heterogeneous mixtures based on the size of the particles. Ordinary mechanical mixtures have parts big enough to see and they stay mixed. E.g. granite Suspensions are large mix ...
Monodisperse FePt Nanoparticles and Ferromagnetic FePt
... Superconducting quantum interference device magnetometry measurements of 4-nm FePt particles show that the as-synthesized particle assemblies are superparamagnetic (coercivity Hc ⫽ 0 Oe) at room temperature. The temperature-dependent magnetization was measured in a 10-Oe field between 5 and 400 K wi ...
... Superconducting quantum interference device magnetometry measurements of 4-nm FePt particles show that the as-synthesized particle assemblies are superparamagnetic (coercivity Hc ⫽ 0 Oe) at room temperature. The temperature-dependent magnetization was measured in a 10-Oe field between 5 and 400 K wi ...
Lowering of the L10 ordering temperature of FePt
... a micellar technique on Si substrates. The phase transition of these magnetic particles towards the chemically ordered L10 phase is tracked for 350 kV He+ ion irradiated samples and compared to a nonirradiated reference. Due to the large separation of the magnetically decoupled particles the array c ...
... a micellar technique on Si substrates. The phase transition of these magnetic particles towards the chemically ordered L10 phase is tracked for 350 kV He+ ion irradiated samples and compared to a nonirradiated reference. Due to the large separation of the magnetically decoupled particles the array c ...
7R CHEMISTRY 1 REVIEW
... 2. If an element is divided into smaller and smaller parts, the smallest particle obtained would be a (an) A) molecule. B) compound. C) mixture. D) atom. 3. The fact that iron cannot be changed into a simpler form indicates that iron is a (an) A) compound. B) molecule. C) element. ...
... 2. If an element is divided into smaller and smaller parts, the smallest particle obtained would be a (an) A) molecule. B) compound. C) mixture. D) atom. 3. The fact that iron cannot be changed into a simpler form indicates that iron is a (an) A) compound. B) molecule. C) element. ...
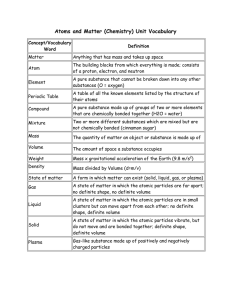
Print › Honors Chemistry Unit 02 Vocabulary | Quizlet
... Honors Chemistry Unit 02 Vocabulary Study online at quizlet.com/_2i1qo4 ...
... Honors Chemistry Unit 02 Vocabulary Study online at quizlet.com/_2i1qo4 ...
1. a) 25% b)86% 2. For my opinion, I think the way to make
... light absorbed can be linearly correlated to the concentration of analyte present. Free atoms of most elements can be produced from samples by the application of high temperatures. In GFAAS, samples are deposited in a small graphite or pyrolytic carbon coated graphite tube, which can then be heated ...
... light absorbed can be linearly correlated to the concentration of analyte present. Free atoms of most elements can be produced from samples by the application of high temperatures. In GFAAS, samples are deposited in a small graphite or pyrolytic carbon coated graphite tube, which can then be heated ...
Classification of Matter
... Detecting Colloids – Pass a beam of light through it Colloid – See beam Solution – Cannot see beam – Particles big enough to scatter light – ...
... Detecting Colloids – Pass a beam of light through it Colloid – See beam Solution – Cannot see beam – Particles big enough to scatter light – ...
File
... element: a substance that cannot be separated or broken down into simpler substances by chemical means. compound: a substance made up of atoms of two ore more different elements joined by chemical bonds. atomic number: the number of protons in the nucleus of an atom. (The atomic number is the same f ...
... element: a substance that cannot be separated or broken down into simpler substances by chemical means. compound: a substance made up of atoms of two ore more different elements joined by chemical bonds. atomic number: the number of protons in the nucleus of an atom. (The atomic number is the same f ...
NAME PERIOD ______ DATE MID-TERM STUDY GUIDE 6.0/HP
... 14. In which statement does Shara state her hypothesis? ________B_______ 15. What is the control in the experiment? _________________D_________________________________ 16. What is the independent variable in this experiment? ___Amount of sugar___________________ 17. What is the dependent variable in ...
... 14. In which statement does Shara state her hypothesis? ________B_______ 15. What is the control in the experiment? _________________D_________________________________ 16. What is the independent variable in this experiment? ___Amount of sugar___________________ 17. What is the dependent variable in ...
history of the atom ppt student copy
... -modified CRT with poles (magnetic field) to attract cathode rays. - passed electricity through a gas at first; then used several samples of other elements. -behavior was same for all elements - rays were attracted to the anode (+). (__________________________) - Concluded that _____________________ ...
... -modified CRT with poles (magnetic field) to attract cathode rays. - passed electricity through a gas at first; then used several samples of other elements. -behavior was same for all elements - rays were attracted to the anode (+). (__________________________) - Concluded that _____________________ ...
Scale, structure and behaviour
... materials may differ from macroscale materials – Gravitational forces become negligible and electromagnetic forces dominate – Quantum mechanics is the model used to describe motion and energy instead of the classical mechanics model – Greater surface area to volume ratios – Random molecular motion b ...
... materials may differ from macroscale materials – Gravitational forces become negligible and electromagnetic forces dominate – Quantum mechanics is the model used to describe motion and energy instead of the classical mechanics model – Greater surface area to volume ratios – Random molecular motion b ...
Chemical Principles – by Steven Zumdahl (5 ) Chapter 1
... Substance is a material that cannot be separated by physical means into two or more materials. (with different properties) Elements are substances that cannot be decomposed by chemical reactions. Elements (atoms) are the simplest forms of matter (in chemistry). Each element is represented by a symbo ...
... Substance is a material that cannot be separated by physical means into two or more materials. (with different properties) Elements are substances that cannot be decomposed by chemical reactions. Elements (atoms) are the simplest forms of matter (in chemistry). Each element is represented by a symbo ...
VOCABULARY name, date, hour: Fill in the number of each term
... ___ substance that is a mixture of two or more metals ___ columns of the periodic table; also known as groups ___ number of protons carried by the nucleus of an atom ___ element with an imbalance in the number of neutrons and protons ___ uncharged particle found in the nucleus of an atom ___ physica ...
... ___ substance that is a mixture of two or more metals ___ columns of the periodic table; also known as groups ___ number of protons carried by the nucleus of an atom ___ element with an imbalance in the number of neutrons and protons ___ uncharged particle found in the nucleus of an atom ___ physica ...
topic 1 sol review homework
... 2. The mass of the calcium atom is due primarily to the mass of its a) protons only b) neutrons only c) protons and neutrons d) protons and electrons 3. Which structure represents a nonpolar molecule? a) H – Cl b) H – H c) H – :O: – H d) NaBr 4. In an aqueous solution, which substance yields hydroge ...
... 2. The mass of the calcium atom is due primarily to the mass of its a) protons only b) neutrons only c) protons and neutrons d) protons and electrons 3. Which structure represents a nonpolar molecule? a) H – Cl b) H – H c) H – :O: – H d) NaBr 4. In an aqueous solution, which substance yields hydroge ...
03 nanoparticles part 7 File - e-learning
... the possibility to produce metals and semiconductors. Disadvantages are hard aggregates and huge aerosol formation. ...
... the possibility to produce metals and semiconductors. Disadvantages are hard aggregates and huge aerosol formation. ...
Honors Midterm - Stamford High School
... 15. What is an isotope? Isotopes of an element have different numbers of neutrons, they also have different mass numbers 16) What are radioisotopes? Radioisotopes can be used to diagnose medical problems and, in some cases, to treat diseases. Background information:. How do you balance nuclear react ...
... 15. What is an isotope? Isotopes of an element have different numbers of neutrons, they also have different mass numbers 16) What are radioisotopes? Radioisotopes can be used to diagnose medical problems and, in some cases, to treat diseases. Background information:. How do you balance nuclear react ...
Elements, Compounds and Mixtures.
... • Pure substance that cannot be separated into simpler substances by physical or chemical means. – Pure substance- a substance in which there is only one type of particle (atom or molecule) ...
... • Pure substance that cannot be separated into simpler substances by physical or chemical means. – Pure substance- a substance in which there is only one type of particle (atom or molecule) ...
E:\My Documents\snc1d\feb12notes.wpd
... The fact that we can turn one pure substance into another new substance tells us that it is possible, with a chemical reaction, to change one type of particle into another! Do we understand how this works yet? No, not in this class anyway. We will have to get a better understanding of the nature of ...
... The fact that we can turn one pure substance into another new substance tells us that it is possible, with a chemical reaction, to change one type of particle into another! Do we understand how this works yet? No, not in this class anyway. We will have to get a better understanding of the nature of ...
Particle-size distribution
The particle-size distribution (PSD) of a powder, or granular material, or particles dispersed in fluid, is a list of values or a mathematical function that defines the relative amount, typically by mass, of particles present according to size. PSD is also known as grain size distribution.