Ch8.Periodic properties
... lowest available energy Hund’s rule: if there are 2 or more orbitals of equal energy (degenerate orbitals), e– will occupy all orbitals singly before pairing ...
... lowest available energy Hund’s rule: if there are 2 or more orbitals of equal energy (degenerate orbitals), e– will occupy all orbitals singly before pairing ...
Motion of a Point Charge in a Magnetic Field
... The time to travel around the circle once turns out to be independent of the size of the circle and the speed. This ends up being very useful in some applications. If there is an electric field present as well, then the net force on a charge q is given by the most general equation, called the Lorent ...
... The time to travel around the circle once turns out to be independent of the size of the circle and the speed. This ends up being very useful in some applications. If there is an electric field present as well, then the net force on a charge q is given by the most general equation, called the Lorent ...
MSWord
... Now, the voltage generated in a loop of wire of area A which is being threaded by this time dependent Bx (t ) field is given by Faraday’s Law: ...
... Now, the voltage generated in a loop of wire of area A which is being threaded by this time dependent Bx (t ) field is given by Faraday’s Law: ...
Chapter 5/6 Notes
... 6.1 Organizing the Elements and Classifying the Elements Origin of the Periodic Table Dimitri Mendeleev – published the first real periodic table in 1869 - Based upon chemical and physical properties - Listed elements in order of increasing atomic mass - Left spaces for undiscovered elements ...
... 6.1 Organizing the Elements and Classifying the Elements Origin of the Periodic Table Dimitri Mendeleev – published the first real periodic table in 1869 - Based upon chemical and physical properties - Listed elements in order of increasing atomic mass - Left spaces for undiscovered elements ...
Exam 3 Solutions - University of Utah Physics
... [45 pts.J A copper rod is sliding on two conducting rails that make an angle of 6 with respect to each other, as in the drawing. The rod is moving to the right with a constant speed v. A uniform magnetic field B is perpendicular to the plane of the paper. (a) ...
... [45 pts.J A copper rod is sliding on two conducting rails that make an angle of 6 with respect to each other, as in the drawing. The rod is moving to the right with a constant speed v. A uniform magnetic field B is perpendicular to the plane of the paper. (a) ...
Electric Field Control of Magnetic Coupling in a Double Quantum
... magnetic) double-exchange. This possibility allows us to create either ferro- or antiferro-correlation between QDs spins, being the prerequisite for construction of universal quantum logic gates [1]. 2. Electric field effects Usually when dealing with the QDs the spherical symmetry is assumed, which ...
... magnetic) double-exchange. This possibility allows us to create either ferro- or antiferro-correlation between QDs spins, being the prerequisite for construction of universal quantum logic gates [1]. 2. Electric field effects Usually when dealing with the QDs the spherical symmetry is assumed, which ...
Dottorato di Ricerca in Fisica dell`Università degli Studi di Messina
... If the building up of smaller and smaller structures promptly answers the recent technological request of more and more miniaturized devices, it is also true that new and exotic features arise below a certain size threshold of particles basically due to quantum confinement of charge carriers (Quantu ...
... If the building up of smaller and smaller structures promptly answers the recent technological request of more and more miniaturized devices, it is also true that new and exotic features arise below a certain size threshold of particles basically due to quantum confinement of charge carriers (Quantu ...
Quantum Dimer Models on the Square Lattice
... the mechanism behind high Tc superconductivity. It is believed that high temperature cuprate superconductors are obtained by doping antiferromagnets with holes or electrons. ...
... the mechanism behind high Tc superconductivity. It is believed that high temperature cuprate superconductors are obtained by doping antiferromagnets with holes or electrons. ...
Part 3. Free Electrons in Metals
... (a) Find the atomic density of sodium. Since it is monovalent, this is the conduction electron density (N/V) in the free electron model. You can do this by noting that this is the same ratio as NA / Vm where NA is the number of atoms per mole (Avogadro’s number) and Vm is the volume per mole (the mo ...
... (a) Find the atomic density of sodium. Since it is monovalent, this is the conduction electron density (N/V) in the free electron model. You can do this by noting that this is the same ratio as NA / Vm where NA is the number of atoms per mole (Avogadro’s number) and Vm is the volume per mole (the mo ...
Prelab01
... Consider the example of the Hydrogen atom; a simplified (but very useful) model for this atom consists of a single electron going in a circular orbit around a single proton. The parameters of the Hydrogen atom are as follows: Electron mass: 9.1 x 10-31 kg; electron charge: -1.6 x 10-19 C; Proton mas ...
... Consider the example of the Hydrogen atom; a simplified (but very useful) model for this atom consists of a single electron going in a circular orbit around a single proton. The parameters of the Hydrogen atom are as follows: Electron mass: 9.1 x 10-31 kg; electron charge: -1.6 x 10-19 C; Proton mas ...
Final Exam, MENA3000 / MENA4000 – Functional Materials, 6
... ε is formed. Cooling through the two fase region more ε is formed whereas liquid become enriched in Sb. At 736 oC residue liquid and all ε crystals transform into FeSb2 via a peritectic reaction. During this reaction, temerature is not changing as the reaction is invariant. After full solidification ...
... ε is formed. Cooling through the two fase region more ε is formed whereas liquid become enriched in Sb. At 736 oC residue liquid and all ε crystals transform into FeSb2 via a peritectic reaction. During this reaction, temerature is not changing as the reaction is invariant. After full solidification ...
Name
... Charged particles at rest are not affected by static magnetic fields. However, when such charged particles are in motion, they are deflected by magnetic fields. The discovery that flowing electrons are affected by magnets was a pivotal discovery at the turn of the 20th century. Today, many common techno ...
... Charged particles at rest are not affected by static magnetic fields. However, when such charged particles are in motion, they are deflected by magnetic fields. The discovery that flowing electrons are affected by magnets was a pivotal discovery at the turn of the 20th century. Today, many common techno ...
Solid - burgess
... 1. Ions are atoms which have gained or lost electrons. 2. examples include Na+, Cl-, Mg2+, O2Try this online quiz on the organization of matter II. Physical and Chemical Properties A. Physical properties 1. properties that are determined by observation, either looking or measuring 2. examples includ ...
... 1. Ions are atoms which have gained or lost electrons. 2. examples include Na+, Cl-, Mg2+, O2Try this online quiz on the organization of matter II. Physical and Chemical Properties A. Physical properties 1. properties that are determined by observation, either looking or measuring 2. examples includ ...
1 Angular momentum and magnetic moment
... A few materials, such as iron, exhibit a more complex phenomenon called ferromagnetism. In this case the imposition of an external magnetic field causes very strong dipole alignment effects to the point where interactions between the newly aligned dipoles prevent the reversion to random orientation ...
... A few materials, such as iron, exhibit a more complex phenomenon called ferromagnetism. In this case the imposition of an external magnetic field causes very strong dipole alignment effects to the point where interactions between the newly aligned dipoles prevent the reversion to random orientation ...
Name
... give the _____________________ of the magnetic field at that point. 9. An object that can attract magnetic materials to itself is called a __________________________________. 10.An object that can be ____________________ or attracted by a magnet is called magnetic. 11.The invisible area around a mag ...
... give the _____________________ of the magnetic field at that point. 9. An object that can attract magnetic materials to itself is called a __________________________________. 10.An object that can be ____________________ or attracted by a magnet is called magnetic. 11.The invisible area around a mag ...
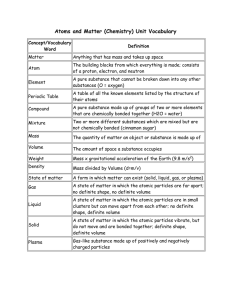
Classification of Matter
... Chemical Properties - Characteristics of a substance that do change the chemical makeup of the substance. How a material reacts or fails to react in the presence of another material to form a ...
... Chemical Properties - Characteristics of a substance that do change the chemical makeup of the substance. How a material reacts or fails to react in the presence of another material to form a ...
Condensed matter physics

Condensed matter physics is a branch of physics that deals with the physical properties of condensed phases of matter. Condensed matter physicists seek to understand the behavior of these phases by using physical laws. In particular, these include the laws of quantum mechanics, electromagnetism and statistical mechanics.The most familiar condensed phases are solids and liquids, while more exotic condensed phases include the superconducting phase exhibited by certain materials at low temperature, the ferromagnetic and antiferromagnetic phases of spins on atomic lattices, and the Bose–Einstein condensate found in cold atomic systems. The study of condensed matter physics involves measuring various material properties via experimental probes along with using techniques of theoretical physics to develop mathematical models that help in understanding physical behavior.The diversity of systems and phenomena available for study makes condensed matter physics the most active field of contemporary physics: one third of all American physicists identify themselves as condensed matter physicists, and the Division of Condensed Matter Physics is the largest division at the American Physical Society. The field overlaps with chemistry, materials science, and nanotechnology, and relates closely to atomic physics and biophysics. Theoretical condensed matter physics shares important concepts and techniques with theoretical particle and nuclear physics.A variety of topics in physics such as crystallography, metallurgy, elasticity, magnetism, etc., were treated as distinct areas, until the 1940s when they were grouped together as solid state physics. Around the 1960s, the study of physical properties of liquids was added to this list, forming the basis for the new, related specialty of condensed matter physics. According to physicist Phil Anderson, the term was coined by him and Volker Heine when they changed the name of their group at the Cavendish Laboratories, Cambridge from ""Solid state theory"" to ""Theory of Condensed Matter"" in 1967, as they felt it did not exclude their interests in the study of liquids, nuclear matter and so on. Although Anderson and Heine helped popularize the name ""condensed matter"", it had been present in Europe for some years, most prominently in the form of a journal published in English, French, and German by Springer-Verlag titled Physics of Condensed Matter, which was launched in 1963. The funding environment and Cold War politics of the 1960s and 1970s were also factors that lead some physicists to prefer the name ""condensed matter physics"", which emphasized the commonality of scientific problems encountered by physicists working on solids, liquids, plasmas, and other complex matter, over ""solid state physics"", which was often associated with the industrial applications of metals and semiconductors. The Bell Telephone Laboratories was one of the first institutes to conduct a research program in condensed matter physics.References to ""condensed"" state can be traced to earlier sources. For example, in the introduction to his 1947 ""Kinetic theory of liquids"" book, Yakov Frenkel proposed that ""The kinetic theory of liquids must accordingly be developed as a generalization and extension of the kinetic theory of solid bodies"". As a matter of fact, it would be more correct to unify them under the title of ""condensed bodies"".