Scanning Tunneling Microscope
... Each plane represents a different value of the tip-sample V, and the lateral position on the plane gives the x,y position of the tip. Filled states are given in red. The plane at the Fermi energy (V=0) is shown in blue. ...
... Each plane represents a different value of the tip-sample V, and the lateral position on the plane gives the x,y position of the tip. Filled states are given in red. The plane at the Fermi energy (V=0) is shown in blue. ...
Magnetic Fields Produced by a Conductors
... Magnetic Fields Produced by Conductors 13.3 and 13.4: The Right Hand Rule and Magnetic Fields of Solenoids ...
... Magnetic Fields Produced by Conductors 13.3 and 13.4: The Right Hand Rule and Magnetic Fields of Solenoids ...
05 Chemistry Basics with Flips 2011
... Two atoms can share more than one pair of electrons double bonds (2 pairs of electrons) triple bonds (3 pairs of electrons) ...
... Two atoms can share more than one pair of electrons double bonds (2 pairs of electrons) triple bonds (3 pairs of electrons) ...
Physical and Chemical Properties
... Chemical Formulas • Chemical formulas show a combination of chemical symbols & numbers that indicate which elements & how many atoms of each element are present in a ...
... Chemical Formulas • Chemical formulas show a combination of chemical symbols & numbers that indicate which elements & how many atoms of each element are present in a ...
chem100c1f
... • A change in the state of matter. It does not involve a change in the substances. E.g. melting of wax and water. • Chemical change: • A change involving at least one of the substances making the matter. E.g. Electrolysis of water, formation of rust: reaction of iron and oxygen to from iron oxide. ...
... • A change in the state of matter. It does not involve a change in the substances. E.g. melting of wax and water. • Chemical change: • A change involving at least one of the substances making the matter. E.g. Electrolysis of water, formation of rust: reaction of iron and oxygen to from iron oxide. ...
CHAPTER 3: The Experimental Basis of Quantum Theory
... In the 1890s scientists and engineers were familiar with “cathode rays”. These rays were generated from one of the metal plates in an evacuated tube across which a large electric potential had been established. It was surmised that cathode rays had something to do with atoms. It was known that catho ...
... In the 1890s scientists and engineers were familiar with “cathode rays”. These rays were generated from one of the metal plates in an evacuated tube across which a large electric potential had been established. It was surmised that cathode rays had something to do with atoms. It was known that catho ...
Name: Mr. Rodriguez
... (a) What is the magnitude of the force per meter of length on a straight wire carrying an 5.40-A current when perpendicular to a 0.97-T uniform magnetic field? (b) What if the angle between the wire and field is 49.0°? ...
... (a) What is the magnitude of the force per meter of length on a straight wire carrying an 5.40-A current when perpendicular to a 0.97-T uniform magnetic field? (b) What if the angle between the wire and field is 49.0°? ...
power point notes
... Rutherford proposed that the atom consists of a tiny positively charged nucleus surrounded by a cloud of negatively charged electrons. The nucleus contains almost all of the mass of the atom and consists of protons and neutrons. The number of electrons surrounding the nucleus, equals the number of p ...
... Rutherford proposed that the atom consists of a tiny positively charged nucleus surrounded by a cloud of negatively charged electrons. The nucleus contains almost all of the mass of the atom and consists of protons and neutrons. The number of electrons surrounding the nucleus, equals the number of p ...
Exam II - Physics
... c. [10 points] Initially unpolarized light passes through two polarizers. First, it is transmitted through a polarizer that is oriented 60.0° with respect to a vertical axis, and then it is transmitted through a polarizer that is oriented at 35.0° with respect to a vertical axis. What fraction of th ...
... c. [10 points] Initially unpolarized light passes through two polarizers. First, it is transmitted through a polarizer that is oriented 60.0° with respect to a vertical axis, and then it is transmitted through a polarizer that is oriented at 35.0° with respect to a vertical axis. What fraction of th ...
Conceptual Questions 1. Compare the kinetic energy gained by a
... the edges), so the electric field strength at both locations A and B will be equal. b) Which point will have the higher electric potential? Explain. The electric potential of a charged particle in a parallel plate apparatus has a linear dependence (V α d) on its distance from the oppositely charged ...
... the edges), so the electric field strength at both locations A and B will be equal. b) Which point will have the higher electric potential? Explain. The electric potential of a charged particle in a parallel plate apparatus has a linear dependence (V α d) on its distance from the oppositely charged ...
Matter and Energy
... Properties of Matter Practice 1. Describe each of the following properties as physical or chemical: a. neon is a color gas at room temperature b. apple slices turn brown when exposed to air c. phosphorus will ignite when exposed to air d. at room temperature, mercury is a liquid e. propane gas is c ...
... Properties of Matter Practice 1. Describe each of the following properties as physical or chemical: a. neon is a color gas at room temperature b. apple slices turn brown when exposed to air c. phosphorus will ignite when exposed to air d. at room temperature, mercury is a liquid e. propane gas is c ...
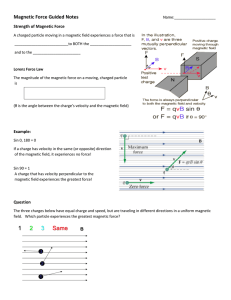
Magnetic Force Guided Notes
... What is the magnitude of the magnetic force on a proton moving at 2.5 X 105 m/s in a magnetic field of 0.5 T … (a) …if the velocity and magnetic field are at right angles? (b) … if the velocity and magnetic field are at 30°? (c) … if the velocity is parallel to a magnetic field? ...
... What is the magnitude of the magnetic force on a proton moving at 2.5 X 105 m/s in a magnetic field of 0.5 T … (a) …if the velocity and magnetic field are at right angles? (b) … if the velocity and magnetic field are at 30°? (c) … if the velocity is parallel to a magnetic field? ...
Atomic Structure - Sakshi Education
... 1. Dalton’s Atomic Theory i. Matter is composed of tiny indivisible particles called atoms which are the building ...
... 1. Dalton’s Atomic Theory i. Matter is composed of tiny indivisible particles called atoms which are the building ...
112 unit II Atom Stru
... weakly attracted to a magnetic field. In these materials there are more electrons of one spin than other and total cancellation does not occur. The extra electrons of one spin cause the atom or the molecule as a whole to behave as if it were itself a tiny magnet. ...
... weakly attracted to a magnetic field. In these materials there are more electrons of one spin than other and total cancellation does not occur. The extra electrons of one spin cause the atom or the molecule as a whole to behave as if it were itself a tiny magnet. ...
Condensed matter physics

Condensed matter physics is a branch of physics that deals with the physical properties of condensed phases of matter. Condensed matter physicists seek to understand the behavior of these phases by using physical laws. In particular, these include the laws of quantum mechanics, electromagnetism and statistical mechanics.The most familiar condensed phases are solids and liquids, while more exotic condensed phases include the superconducting phase exhibited by certain materials at low temperature, the ferromagnetic and antiferromagnetic phases of spins on atomic lattices, and the Bose–Einstein condensate found in cold atomic systems. The study of condensed matter physics involves measuring various material properties via experimental probes along with using techniques of theoretical physics to develop mathematical models that help in understanding physical behavior.The diversity of systems and phenomena available for study makes condensed matter physics the most active field of contemporary physics: one third of all American physicists identify themselves as condensed matter physicists, and the Division of Condensed Matter Physics is the largest division at the American Physical Society. The field overlaps with chemistry, materials science, and nanotechnology, and relates closely to atomic physics and biophysics. Theoretical condensed matter physics shares important concepts and techniques with theoretical particle and nuclear physics.A variety of topics in physics such as crystallography, metallurgy, elasticity, magnetism, etc., were treated as distinct areas, until the 1940s when they were grouped together as solid state physics. Around the 1960s, the study of physical properties of liquids was added to this list, forming the basis for the new, related specialty of condensed matter physics. According to physicist Phil Anderson, the term was coined by him and Volker Heine when they changed the name of their group at the Cavendish Laboratories, Cambridge from ""Solid state theory"" to ""Theory of Condensed Matter"" in 1967, as they felt it did not exclude their interests in the study of liquids, nuclear matter and so on. Although Anderson and Heine helped popularize the name ""condensed matter"", it had been present in Europe for some years, most prominently in the form of a journal published in English, French, and German by Springer-Verlag titled Physics of Condensed Matter, which was launched in 1963. The funding environment and Cold War politics of the 1960s and 1970s were also factors that lead some physicists to prefer the name ""condensed matter physics"", which emphasized the commonality of scientific problems encountered by physicists working on solids, liquids, plasmas, and other complex matter, over ""solid state physics"", which was often associated with the industrial applications of metals and semiconductors. The Bell Telephone Laboratories was one of the first institutes to conduct a research program in condensed matter physics.References to ""condensed"" state can be traced to earlier sources. For example, in the introduction to his 1947 ""Kinetic theory of liquids"" book, Yakov Frenkel proposed that ""The kinetic theory of liquids must accordingly be developed as a generalization and extension of the kinetic theory of solid bodies"". As a matter of fact, it would be more correct to unify them under the title of ""condensed bodies"".