problems
... radius R. Imagine the disk divided into rings of varying radii r, as shown. Show that the net electric field on the disk axis has magnitude ...
... radius R. Imagine the disk divided into rings of varying radii r, as shown. Show that the net electric field on the disk axis has magnitude ...
Classification of Matter
... mixtures are the “least mixed” of all mixtures The different particles in mixtures are large enough to be seen ...
... mixtures are the “least mixed” of all mixtures The different particles in mixtures are large enough to be seen ...
Milikan`s Oil Drop Experiment
... the changes in the charge were always a multiple of –1.6x10-19 C. The changes were caused by one or more electrons being added to or removed from the drops. He concluded that the smallest change in charge that could occur was the amount of charge of one electron. Therefore, Milikan said that each el ...
... the changes in the charge were always a multiple of –1.6x10-19 C. The changes were caused by one or more electrons being added to or removed from the drops. He concluded that the smallest change in charge that could occur was the amount of charge of one electron. Therefore, Milikan said that each el ...
Quantum critical point and spin fluctuations in the lower
... of Fe2+ at the critical pressure Pc of the spin transition and close to T=0 is determined by a quantum critical point Pq (T = 0, Pc) where the energy difference between the HS and LS states (an energy gap for the spin fluctuation) is zero. The deviation from T=0 leads to the thermal excitation for t ...
... of Fe2+ at the critical pressure Pc of the spin transition and close to T=0 is determined by a quantum critical point Pq (T = 0, Pc) where the energy difference between the HS and LS states (an energy gap for the spin fluctuation) is zero. The deviation from T=0 leads to the thermal excitation for t ...
Document
... Most ceramics have very high Tb. Consequently their vapor pressures are negligible at room temperature and become appreciable only at high temperature. Also very few ceramics vaporize without a molecular change. The result is that the vapor composition is usually not the same as that of the original ...
... Most ceramics have very high Tb. Consequently their vapor pressures are negligible at room temperature and become appreciable only at high temperature. Also very few ceramics vaporize without a molecular change. The result is that the vapor composition is usually not the same as that of the original ...
Electrical Conductivity
... occupied & unoccupied k states at absolute zero for the free electron gas. ...
... occupied & unoccupied k states at absolute zero for the free electron gas. ...
Hierarchy of Planck Constants
... constant (see this and this). Effects of ELF em fields on vertebrate brain quantal although energies of the photons extremely small as compared to thermal energy for ordinary value of Planck constant (see this). Could hbar be quantized and have arbitrarily large values? Possible only if the notion o ...
... constant (see this and this). Effects of ELF em fields on vertebrate brain quantal although energies of the photons extremely small as compared to thermal energy for ordinary value of Planck constant (see this). Could hbar be quantized and have arbitrarily large values? Possible only if the notion o ...
Faraday`s Law - Power Shaver
... When a magnet is moved into a coil of wire, changing the magnetic field and magnetic flux through the coil, a voltage will be generated in the coil according to Faraday's Law. In the example shown below, when the magnet is moved into the coil the galvanometer deflects to the left in response to the ...
... When a magnet is moved into a coil of wire, changing the magnetic field and magnetic flux through the coil, a voltage will be generated in the coil according to Faraday's Law. In the example shown below, when the magnet is moved into the coil the galvanometer deflects to the left in response to the ...
ionization energies
... • As more and more elements were discovered, chemists began to notice patterns in the chemical properties of certain elements. • Consider the three metals Li, Na, and K • All 3 metals are soft • All 3 metals are less dense than water • All 3 metals have similar appearance and low melting points • Th ...
... • As more and more elements were discovered, chemists began to notice patterns in the chemical properties of certain elements. • Consider the three metals Li, Na, and K • All 3 metals are soft • All 3 metals are less dense than water • All 3 metals have similar appearance and low melting points • Th ...
Document
... The first microscopic attempt to model electrical conductivity in metals was made by P. Drude (1900) – He considered the valence electrons to be a dense classical gas ...
... The first microscopic attempt to model electrical conductivity in metals was made by P. Drude (1900) – He considered the valence electrons to be a dense classical gas ...
Objective 4
... beakers. The first solution has a pH of 4, and the pH of the second solution is unknown. If the two solutions are mixed and the resulting pH is 5, the second solution must have — A fewer suspended solids B a lower temperature C more dissolved salt (NaCl) particles D a higher concentration of OH– ion ...
... beakers. The first solution has a pH of 4, and the pH of the second solution is unknown. If the two solutions are mixed and the resulting pH is 5, the second solution must have — A fewer suspended solids B a lower temperature C more dissolved salt (NaCl) particles D a higher concentration of OH– ion ...
ELECTRON SPIN RESONANCE SPECTROCOPY
... electronic magnetic moments relative to applied magnetic filed and the transition between these energy states occurs on the application of an appropriate frequency in the ...
... electronic magnetic moments relative to applied magnetic filed and the transition between these energy states occurs on the application of an appropriate frequency in the ...
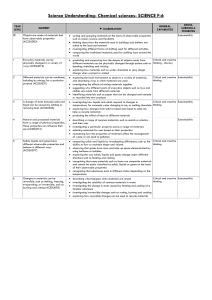
Chemical sciences- SCIENCE F-6
... Critical and creative predicting and comparing how the shapes of objects made from different materials can be physically changed through actions such as thinking , Literacy bending, stretching and twisting exploring how materials such as water, chocolate or play dough change when warmed or coole ...
... Critical and creative predicting and comparing how the shapes of objects made from different materials can be physically changed through actions such as thinking , Literacy bending, stretching and twisting exploring how materials such as water, chocolate or play dough change when warmed or coole ...
Condensed matter physics

Condensed matter physics is a branch of physics that deals with the physical properties of condensed phases of matter. Condensed matter physicists seek to understand the behavior of these phases by using physical laws. In particular, these include the laws of quantum mechanics, electromagnetism and statistical mechanics.The most familiar condensed phases are solids and liquids, while more exotic condensed phases include the superconducting phase exhibited by certain materials at low temperature, the ferromagnetic and antiferromagnetic phases of spins on atomic lattices, and the Bose–Einstein condensate found in cold atomic systems. The study of condensed matter physics involves measuring various material properties via experimental probes along with using techniques of theoretical physics to develop mathematical models that help in understanding physical behavior.The diversity of systems and phenomena available for study makes condensed matter physics the most active field of contemporary physics: one third of all American physicists identify themselves as condensed matter physicists, and the Division of Condensed Matter Physics is the largest division at the American Physical Society. The field overlaps with chemistry, materials science, and nanotechnology, and relates closely to atomic physics and biophysics. Theoretical condensed matter physics shares important concepts and techniques with theoretical particle and nuclear physics.A variety of topics in physics such as crystallography, metallurgy, elasticity, magnetism, etc., were treated as distinct areas, until the 1940s when they were grouped together as solid state physics. Around the 1960s, the study of physical properties of liquids was added to this list, forming the basis for the new, related specialty of condensed matter physics. According to physicist Phil Anderson, the term was coined by him and Volker Heine when they changed the name of their group at the Cavendish Laboratories, Cambridge from ""Solid state theory"" to ""Theory of Condensed Matter"" in 1967, as they felt it did not exclude their interests in the study of liquids, nuclear matter and so on. Although Anderson and Heine helped popularize the name ""condensed matter"", it had been present in Europe for some years, most prominently in the form of a journal published in English, French, and German by Springer-Verlag titled Physics of Condensed Matter, which was launched in 1963. The funding environment and Cold War politics of the 1960s and 1970s were also factors that lead some physicists to prefer the name ""condensed matter physics"", which emphasized the commonality of scientific problems encountered by physicists working on solids, liquids, plasmas, and other complex matter, over ""solid state physics"", which was often associated with the industrial applications of metals and semiconductors. The Bell Telephone Laboratories was one of the first institutes to conduct a research program in condensed matter physics.References to ""condensed"" state can be traced to earlier sources. For example, in the introduction to his 1947 ""Kinetic theory of liquids"" book, Yakov Frenkel proposed that ""The kinetic theory of liquids must accordingly be developed as a generalization and extension of the kinetic theory of solid bodies"". As a matter of fact, it would be more correct to unify them under the title of ""condensed bodies"".