Electronic structure of mixed valence transition metal oxides
... Convenient and sensitive tool to study the defects and impurities in ferrites proved to be the nuclear magnetic resonance. As we demonstrated on numerous examples (see [6] for a review) the presence of an impurity leads to a modification of the hyperfine field on the nuclei of neighboring Fe ions. ...
... Convenient and sensitive tool to study the defects and impurities in ferrites proved to be the nuclear magnetic resonance. As we demonstrated on numerous examples (see [6] for a review) the presence of an impurity leads to a modification of the hyperfine field on the nuclei of neighboring Fe ions. ...
Week 4 AB - Help-A-Bull
... Thermal conduction in a metal involves transferring energy from the hot region to the cold region by conduction electrons. More energetic electrons (shown with longer velocity vectors) from the hotter regions arrive at cooler regions and collide there with lattice vibrations and transfer their energ ...
... Thermal conduction in a metal involves transferring energy from the hot region to the cold region by conduction electrons. More energetic electrons (shown with longer velocity vectors) from the hotter regions arrive at cooler regions and collide there with lattice vibrations and transfer their energ ...
Advancements in Electromagnetic Material Properties
... Paramagnetic materials are also characterized by their weak atomic moments. In a paramagnetic material, each atom already has an intrinsic (and permanent) magnetic dipole moment, but these atoms are oriented randomly and so give the material a net moment of zero. If, however, an external magnetic fi ...
... Paramagnetic materials are also characterized by their weak atomic moments. In a paramagnetic material, each atom already has an intrinsic (and permanent) magnetic dipole moment, but these atoms are oriented randomly and so give the material a net moment of zero. If, however, an external magnetic fi ...
Dr. Audrey Lugo`s AP Chemistry Course Syllabus
... These descriptive facts, including the chemistry involved in environmental and societal issues, should not be isolated from the principles being studied but should be taught throughout the course to illustrate and illuminate the principles. The following areas should be covered: 1. Chemical reactivi ...
... These descriptive facts, including the chemistry involved in environmental and societal issues, should not be isolated from the principles being studied but should be taught throughout the course to illustrate and illuminate the principles. The following areas should be covered: 1. Chemical reactivi ...
Chapter 31: Faraday`s Law
... This section derives a formula for the emf induced in a straight segment of wire moving through a constant magnetic field: X œ FP@ Here F is the magnetic field, P is the length of the segment, and @ is the velocity of the segment. This formula only works for a straight wire, and it assumes that the ...
... This section derives a formula for the emf induced in a straight segment of wire moving through a constant magnetic field: X œ FP@ Here F is the magnetic field, P is the length of the segment, and @ is the velocity of the segment. This formula only works for a straight wire, and it assumes that the ...
The ocean is a mixture.
... Atoms of this family have 6 valence electrons. Most elements in this family share electrons when forming compounds. Oxygen is the most abundant element in the earth’s crust. It is extremely active and combines with almost all elements. Reactivity: Varies among the elements Properties: All but nitrog ...
... Atoms of this family have 6 valence electrons. Most elements in this family share electrons when forming compounds. Oxygen is the most abundant element in the earth’s crust. It is extremely active and combines with almost all elements. Reactivity: Varies among the elements Properties: All but nitrog ...
File
... a) What is happening during a plateau in the graph? b) What is happening when the line is going up or down? c) Be able to identify the phase change on the graph? d) Which phase changes are endothermic? Why? e) Which phase changes are exothermic? Why? ...
... a) What is happening during a plateau in the graph? b) What is happening when the line is going up or down? c) Be able to identify the phase change on the graph? d) Which phase changes are endothermic? Why? e) Which phase changes are exothermic? Why? ...
Annotation of all the Homework Problems describing which ones to
... susceptibility of the conduction electron in metals. Here susceptibility is χ = dM/dH = µ0 dM/dB at small H and is meant to consider the magnetization of the electron spins only. (c) How are the results of (a) and (b) different from that of a classical gas of electrons? What other properties of meta ...
... susceptibility of the conduction electron in metals. Here susceptibility is χ = dM/dH = µ0 dM/dB at small H and is meant to consider the magnetization of the electron spins only. (c) How are the results of (a) and (b) different from that of a classical gas of electrons? What other properties of meta ...
Electrical Properties
... by impurities, they are called extrinsic semiconductors. In semiconductors, the valence and conduction bands do not overlap as in metals, but they possess enough electrons in the valence band those can be promoted to the conduction band at a certain temperature. ...
... by impurities, they are called extrinsic semiconductors. In semiconductors, the valence and conduction bands do not overlap as in metals, but they possess enough electrons in the valence band those can be promoted to the conduction band at a certain temperature. ...
worksheer format 11-12
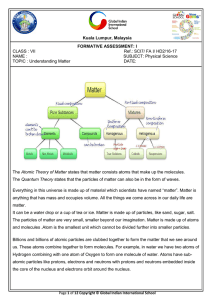
... being solid, liquid, gas, plasma and a new one called Bose-Einstein condensates.. As of 1995, scientists have identified five states of matter.Matter can change states as well as participate in chemical changes, depending on their chemical properties and composition. ...
... being solid, liquid, gas, plasma and a new one called Bose-Einstein condensates.. As of 1995, scientists have identified five states of matter.Matter can change states as well as participate in chemical changes, depending on their chemical properties and composition. ...
Condensed matter physics

Condensed matter physics is a branch of physics that deals with the physical properties of condensed phases of matter. Condensed matter physicists seek to understand the behavior of these phases by using physical laws. In particular, these include the laws of quantum mechanics, electromagnetism and statistical mechanics.The most familiar condensed phases are solids and liquids, while more exotic condensed phases include the superconducting phase exhibited by certain materials at low temperature, the ferromagnetic and antiferromagnetic phases of spins on atomic lattices, and the Bose–Einstein condensate found in cold atomic systems. The study of condensed matter physics involves measuring various material properties via experimental probes along with using techniques of theoretical physics to develop mathematical models that help in understanding physical behavior.The diversity of systems and phenomena available for study makes condensed matter physics the most active field of contemporary physics: one third of all American physicists identify themselves as condensed matter physicists, and the Division of Condensed Matter Physics is the largest division at the American Physical Society. The field overlaps with chemistry, materials science, and nanotechnology, and relates closely to atomic physics and biophysics. Theoretical condensed matter physics shares important concepts and techniques with theoretical particle and nuclear physics.A variety of topics in physics such as crystallography, metallurgy, elasticity, magnetism, etc., were treated as distinct areas, until the 1940s when they were grouped together as solid state physics. Around the 1960s, the study of physical properties of liquids was added to this list, forming the basis for the new, related specialty of condensed matter physics. According to physicist Phil Anderson, the term was coined by him and Volker Heine when they changed the name of their group at the Cavendish Laboratories, Cambridge from ""Solid state theory"" to ""Theory of Condensed Matter"" in 1967, as they felt it did not exclude their interests in the study of liquids, nuclear matter and so on. Although Anderson and Heine helped popularize the name ""condensed matter"", it had been present in Europe for some years, most prominently in the form of a journal published in English, French, and German by Springer-Verlag titled Physics of Condensed Matter, which was launched in 1963. The funding environment and Cold War politics of the 1960s and 1970s were also factors that lead some physicists to prefer the name ""condensed matter physics"", which emphasized the commonality of scientific problems encountered by physicists working on solids, liquids, plasmas, and other complex matter, over ""solid state physics"", which was often associated with the industrial applications of metals and semiconductors. The Bell Telephone Laboratories was one of the first institutes to conduct a research program in condensed matter physics.References to ""condensed"" state can be traced to earlier sources. For example, in the introduction to his 1947 ""Kinetic theory of liquids"" book, Yakov Frenkel proposed that ""The kinetic theory of liquids must accordingly be developed as a generalization and extension of the kinetic theory of solid bodies"". As a matter of fact, it would be more correct to unify them under the title of ""condensed bodies"".