Henry Moseley, the Atomic Number, and Synthesis
... to advance the understanding of the elements and solve the problem with Mendeleev’s periodic table. Explain that organizing the elements by their weight did not always give a periodic alignment of their chemical properties. Moseley noticed that shooting electrons at elements caused them to release x ...
... to advance the understanding of the elements and solve the problem with Mendeleev’s periodic table. Explain that organizing the elements by their weight did not always give a periodic alignment of their chemical properties. Moseley noticed that shooting electrons at elements caused them to release x ...
Henry Moseley, the Atomic Number, and Synthesis
... to advance the understanding of the elements and solve the problem with Mendeleev’s periodic table. Explain that organizing the elements by their weight did not always give a periodic alignment of their chemical properties. Moseley noticed that shooting electrons at elements caused them to release x ...
... to advance the understanding of the elements and solve the problem with Mendeleev’s periodic table. Explain that organizing the elements by their weight did not always give a periodic alignment of their chemical properties. Moseley noticed that shooting electrons at elements caused them to release x ...
AP CHEMISTRY SUMMER ASSIGNMENT AP Chemistry is a
... 9. How many Energy levels do strontium and iodine have? ________ 10. Which element is located in Period 4, group 2? ________________ 11. Name an element that has similar properties to sodium. __________ 12. State a property of cobalt._______________________________________ 13. Cobalt has a total of ...
... 9. How many Energy levels do strontium and iodine have? ________ 10. Which element is located in Period 4, group 2? ________________ 11. Name an element that has similar properties to sodium. __________ 12. State a property of cobalt._______________________________________ 13. Cobalt has a total of ...
specific vocabulary of the unit
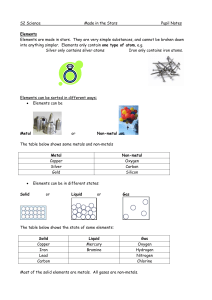
... non-metals on the Periodic Table of Elements. They are sometimes called semi-metals and have characteristics that resemble both metals and nonmetals. ...
... non-metals on the Periodic Table of Elements. They are sometimes called semi-metals and have characteristics that resemble both metals and nonmetals. ...
Goal 4.01
... Given the notation of an element you should be able to determine the number of p, n, and e. The first step is to find the element on the periodic table and determine its atomic number which gives you the number of p. The number of p’s will never change. From there you must determine the number of n ...
... Given the notation of an element you should be able to determine the number of p, n, and e. The first step is to find the element on the periodic table and determine its atomic number which gives you the number of p. The number of p’s will never change. From there you must determine the number of n ...
Structure - Mole Cafe
... Atoms are solid, homogeneous, indestructible, and indivisible Different atoms have different sizes and shapes The differing properties of matter are due to the size, shape, and movement of atoms Changes in matter result from changes in the groupings of atoms and not the atoms ...
... Atoms are solid, homogeneous, indestructible, and indivisible Different atoms have different sizes and shapes The differing properties of matter are due to the size, shape, and movement of atoms Changes in matter result from changes in the groupings of atoms and not the atoms ...
Chapter 1 Notes
... • Early scientists theorized that eventually you would not be able to cut it in half any more. o Only one particle would be left. o They named these particles ‘Atoms’ • Atoms means ‘cannot be divided’ • Scientists could not study this because they lacked the tools to see things this small. ...
... • Early scientists theorized that eventually you would not be able to cut it in half any more. o Only one particle would be left. o They named these particles ‘Atoms’ • Atoms means ‘cannot be divided’ • Scientists could not study this because they lacked the tools to see things this small. ...
Made in the Stars Notes
... at room temperature except for mercury, which is a liquid. Non-metal solids are usually brittle (they break easily). Non-metals can be solids, liquids or gases at room temperature. Non-metals usually have low melting and boiling points. They are poor conductors of electricity. The exception is graph ...
... at room temperature except for mercury, which is a liquid. Non-metal solids are usually brittle (they break easily). Non-metals can be solids, liquids or gases at room temperature. Non-metals usually have low melting and boiling points. They are poor conductors of electricity. The exception is graph ...
3.2 Notes
... Scientists have identified 109 different __________________________________ o 90 types are found in ____________________________ o Remaining 19 are _____________________________________________ o Represented by a _____________________________________________ ...
... Scientists have identified 109 different __________________________________ o 90 types are found in ____________________________ o Remaining 19 are _____________________________________________ o Represented by a _____________________________________________ ...
the Atom
... tiny, indivisible, indestructible particles. Each one has a certain mass, size, and chemical behavior that was determined by what kind of element they were. A Summary of Dalton’s Atomic Theory: ...
... tiny, indivisible, indestructible particles. Each one has a certain mass, size, and chemical behavior that was determined by what kind of element they were. A Summary of Dalton’s Atomic Theory: ...
ps-5-1-and-5-2-ws
... 16.The average mass of all the isotopes of an element is called the ___________________________ 17.The modern periodic table is arranged in order of increasing _______________________________ 18. Information found on the periodic table for each element includes its atomic number, , name, and atomic ...
... 16.The average mass of all the isotopes of an element is called the ___________________________ 17.The modern periodic table is arranged in order of increasing _______________________________ 18. Information found on the periodic table for each element includes its atomic number, , name, and atomic ...
Isotopes-Chemistry
... Same Element Different AtomIsotopes All atoms of a particular element are not exactly alike. Some elements have atoms with different masses (isotopes) ...
... Same Element Different AtomIsotopes All atoms of a particular element are not exactly alike. Some elements have atoms with different masses (isotopes) ...
Name Date Period DEFINING THE ATOM Section Review
... Part B True-False Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT. 5. Atoms of one element change into atoms of another element during chemical reactions. 6. Atoms combine in one-to-one ratios to form compounds. 7. Atoms of one element are different from a ...
... Part B True-False Classify each of these statements as always true, AT; sometimes true, ST; or never true, NT. 5. Atoms of one element change into atoms of another element during chemical reactions. 6. Atoms combine in one-to-one ratios to form compounds. 7. Atoms of one element are different from a ...
Review Questions 1. How many protons does potassium have? 2
... 1. How many protons does potassium have? 2. How many protons would the element directly to the right of strontium have? Match the common name and symbol of the element on the left with the name from which the symbol was derived on the right. 3. antimony, Sb 4. tin, Sn 5. sodium, Na 6. lead, Pb 7. tu ...
... 1. How many protons does potassium have? 2. How many protons would the element directly to the right of strontium have? Match the common name and symbol of the element on the left with the name from which the symbol was derived on the right. 3. antimony, Sb 4. tin, Sn 5. sodium, Na 6. lead, Pb 7. tu ...
Chapter 2: Atoms, Molecules, and Ions
... Exam Problem. A sample of ascorbic acid (vitamin C) is synthesized in the laboratory. It contains 1.50 g of carbon and 2.00 g of oxygen. Another sample of ascorbic acid isolated from citrus fruits contains 9.22 g of carbon. How many grams of oxygen does it contain? ...
... Exam Problem. A sample of ascorbic acid (vitamin C) is synthesized in the laboratory. It contains 1.50 g of carbon and 2.00 g of oxygen. Another sample of ascorbic acid isolated from citrus fruits contains 9.22 g of carbon. How many grams of oxygen does it contain? ...
Atomic Structure Worksheet
... Look at the atomic weights of a few different elements on your periodic table. Do you notice that very few of the elements have atomic weights that are close to being nice whole numbers? Do you know why this is? After all, for our purposes, the mass of both the proton and the neutron are almost exac ...
... Look at the atomic weights of a few different elements on your periodic table. Do you notice that very few of the elements have atomic weights that are close to being nice whole numbers? Do you know why this is? After all, for our purposes, the mass of both the proton and the neutron are almost exac ...
Atomic Structure Notes
... How heavy is an atom of oxygen? It depends, because there are different kinds of oxygen atoms. We are more concerned with the average atomic mass. This is based on the abundance (percentage) of each variety of that element in nature. ...
... How heavy is an atom of oxygen? It depends, because there are different kinds of oxygen atoms. We are more concerned with the average atomic mass. This is based on the abundance (percentage) of each variety of that element in nature. ...
Atomic Structure
... When scientists design models of atoms, they usually show a simplified version of the atom's nucleus and its subatomic particles. The nucleus is made up of protons and neutrons (picture red and blue gumballs stuck together) with electrons moving at high speeds around the outside of the nucleus (imag ...
... When scientists design models of atoms, they usually show a simplified version of the atom's nucleus and its subatomic particles. The nucleus is made up of protons and neutrons (picture red and blue gumballs stuck together) with electrons moving at high speeds around the outside of the nucleus (imag ...
Atoms, Molecules and Ions
... • Dalton’s basic postulates (ideas) were: – All matter is composed of atoms which are indivisible and indestructible. Atoms are considered as the ultimate chemical particles. – An element is composed of identical atoms with fixed, identical properties and masses. – Compounds are formed by the combin ...
... • Dalton’s basic postulates (ideas) were: – All matter is composed of atoms which are indivisible and indestructible. Atoms are considered as the ultimate chemical particles. – An element is composed of identical atoms with fixed, identical properties and masses. – Compounds are formed by the combin ...
atomic number - Net Start Class
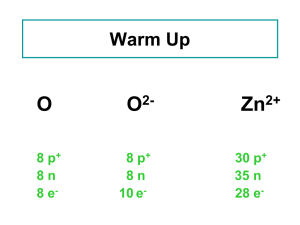
... • An atom with the same number of protons but different number of neutrons are called isotopes. • Isotopes are chemically alike, because it is the protons which are responsible for the chemical behavior. ...
... • An atom with the same number of protons but different number of neutrons are called isotopes. • Isotopes are chemically alike, because it is the protons which are responsible for the chemical behavior. ...