Introduction to Antimicrobial Therapy
... therapy. Surgical intervention. Vancomycin discontinued. ...
... therapy. Surgical intervention. Vancomycin discontinued. ...
A Practical Guide to Dermatological Drug Use in Pregnancy
... safest therapy for extensive psoriasis during pregnancy, although overheating during treatment should be avoided. PUVA is a potential teratogen because it is known to be mutagenic and to induce sister chromatid exchanges. However, adverse outcomes have not been reported in studies of women exposed t ...
... safest therapy for extensive psoriasis during pregnancy, although overheating during treatment should be avoided. PUVA is a potential teratogen because it is known to be mutagenic and to induce sister chromatid exchanges. However, adverse outcomes have not been reported in studies of women exposed t ...
Inventory Management Requirements for Controlled Substances
... a third parties reimburse a pharmacy based on the AWP less an agreed on discount. Therefore the pharmacy has an incentive to purchase a drug as far below its AWP as possible. • Capitation Fee: This reimbursement plan is infrequently used because it places all risk on the pharmacy without adequate co ...
... a third parties reimburse a pharmacy based on the AWP less an agreed on discount. Therefore the pharmacy has an incentive to purchase a drug as far below its AWP as possible. • Capitation Fee: This reimbursement plan is infrequently used because it places all risk on the pharmacy without adequate co ...
ProposalForMedsAllergiesLabsSDWG_WG_2012_10_4
... drugs that meet intent but were missed by the value set. We’re still allowing the current NQF value sets to be used as per the original rule design for those reporting entities that just want to use the provided extensional value set because it’s easier for them. CMS would now be able to easily anal ...
... drugs that meet intent but were missed by the value set. We’re still allowing the current NQF value sets to be used as per the original rule design for those reporting entities that just want to use the provided extensional value set because it’s easier for them. CMS would now be able to easily anal ...
BENYLIN CHILDREN’S TICKLY COUGHS SYRUP PL 00014/0645 UKPAR
... Children’s Tickly Coughs Syrup to The Boots Company Plc (trading as BCM) on 21 June 2007. This product is available on the general sale list. The application was submitted as a simple abridged application according to Article 10c of Directive 2001/83/EC as amended, referring to Benylin Children’s Ti ...
... Children’s Tickly Coughs Syrup to The Boots Company Plc (trading as BCM) on 21 June 2007. This product is available on the general sale list. The application was submitted as a simple abridged application according to Article 10c of Directive 2001/83/EC as amended, referring to Benylin Children’s Ti ...
Title goes here - University of Washington
... Guidance Documents have proliferated to aid investigators in understanding the requirements for INDs and IDEs Product and Indication Specific Confidential ...
... Guidance Documents have proliferated to aid investigators in understanding the requirements for INDs and IDEs Product and Indication Specific Confidential ...
Over-the-Counter Medications: A Success Story
... In concluding my portion of our remarks, let me emphasize that self-care is here to stay! Consumers demand it; they are aware of it, and want more control over self-care. Secondly, the switch of drugs from prescription to nonprescription has been phenomenally successful. This success has stemmed fro ...
... In concluding my portion of our remarks, let me emphasize that self-care is here to stay! Consumers demand it; they are aware of it, and want more control over self-care. Secondly, the switch of drugs from prescription to nonprescription has been phenomenally successful. This success has stemmed fro ...
Therapeutic Category Drug Class Clinical Edits
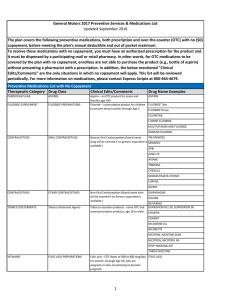
... General Motors 2017 Preventive Services & Medications List Updated September 2016 The plan covers the following preventive medications, both prescription and over-the-counter (OTC) with no ($0) copayment, before meeting the plan’s annual deductible and out of pocket maximum. To receive these medicat ...
... General Motors 2017 Preventive Services & Medications List Updated September 2016 The plan covers the following preventive medications, both prescription and over-the-counter (OTC) with no ($0) copayment, before meeting the plan’s annual deductible and out of pocket maximum. To receive these medicat ...
SECURITIES AND EXCHANGE COMMISSION WASHINGTON, D.C.
... In January 2011, Neuralstem reached a confidential settlement with ReNeuron, Ltd., ending litigation between the parties. ReNeuron agreed to immediately compensate Neuralstem, as well as to make future milestone and royalty payments to Neuralstem based on ReNeuron’s development of certain products ...
... In January 2011, Neuralstem reached a confidential settlement with ReNeuron, Ltd., ending litigation between the parties. ReNeuron agreed to immediately compensate Neuralstem, as well as to make future milestone and royalty payments to Neuralstem based on ReNeuron’s development of certain products ...
Dr. Michael Sinz zpresentation
... • Static nature of in vitro systems compared to the dynamic in vivo system – can lead to misleading conclusions • Mixtures of interaction mechanisms from the same compound are extremely difficult to predict: – reversible + irreversible inhibition – inhibition + induction ...
... • Static nature of in vitro systems compared to the dynamic in vivo system – can lead to misleading conclusions • Mixtures of interaction mechanisms from the same compound are extremely difficult to predict: – reversible + irreversible inhibition – inhibition + induction ...
Frequently Asked Questions
... non-preferred drugs is subject to prior authorization at this time. 15. Are there some therapeutic classes that will be excluded from the PDL PA process? Yes, in general drugs used for the treatment of human immunodeficiency virus or acquired immune deficiency syndrome and cancer are excluded from t ...
... non-preferred drugs is subject to prior authorization at this time. 15. Are there some therapeutic classes that will be excluded from the PDL PA process? Yes, in general drugs used for the treatment of human immunodeficiency virus or acquired immune deficiency syndrome and cancer are excluded from t ...
‘-4 L
... those products are inaccurate and based on inadequate data. You raise concerns that the monographs will inappropriately validate any synthetic conjugated estrogens drug product and mistakenly foster the inference that any such product is the same as Premarin. To support your argument, you cite state ...
... those products are inaccurate and based on inadequate data. You raise concerns that the monographs will inappropriately validate any synthetic conjugated estrogens drug product and mistakenly foster the inference that any such product is the same as Premarin. To support your argument, you cite state ...
sued
... application or which claims a method of using such drug and with respect to which a claim of patent infringement could reasonably be asserted if a person not licensed by the owner engaged in the manufacture, use, or sale of the drug.” Id. § 355(b)(1); see also 21 C.F.R. § 314.50(h) (citing § 314.53( ...
... application or which claims a method of using such drug and with respect to which a claim of patent infringement could reasonably be asserted if a person not licensed by the owner engaged in the manufacture, use, or sale of the drug.” Id. § 355(b)(1); see also 21 C.F.R. § 314.50(h) (citing § 314.53( ...
APR,. 3 2006 Memorandum
... cytotoxicity . Hence, the polymer is considered safe for use in the current system. Lactic acid is a normal body constituent, and is a metabolic product of many drugs, consumer products and endogenous compounds. Although the toxicological database of lactic acid is limited, the results of these demo ...
... cytotoxicity . Hence, the polymer is considered safe for use in the current system. Lactic acid is a normal body constituent, and is a metabolic product of many drugs, consumer products and endogenous compounds. Although the toxicological database of lactic acid is limited, the results of these demo ...
New Twists and Turns in Designer Drug Abuse
... purported that many contemporary salts and cleaners contain a legal stimulant called methylhexaneamine. The Journal has reported on this substance in the past. Its effects are amphetamine-like, at higher doses it purports to have aphrodisiac powers. Readers should keep an eye out for other salt or c ...
... purported that many contemporary salts and cleaners contain a legal stimulant called methylhexaneamine. The Journal has reported on this substance in the past. Its effects are amphetamine-like, at higher doses it purports to have aphrodisiac powers. Readers should keep an eye out for other salt or c ...
Issued: November 2012 AN: 01536/2011 SUMMARY OF PRODUCT
... Fluoroquinolones should be reserved for the treatment of clinical conditions which have responded poorly, or are expected to respond poorly, to other classes of antimicrobials. Whenever possible, fluoroquinolones should only be used based on susceptibility testing. Use of the product deviating from ...
... Fluoroquinolones should be reserved for the treatment of clinical conditions which have responded poorly, or are expected to respond poorly, to other classes of antimicrobials. Whenever possible, fluoroquinolones should only be used based on susceptibility testing. Use of the product deviating from ...
Introduction to Generic Drug Product Development
... • The availability of technology and the cost of acquiring technology to manufacture the product will also impact on the choice of generic drug: • If the technology requires a fluidized bed coater, roller compactor, or any other special equipment, then the firm must consider whether this equipment i ...
... • The availability of technology and the cost of acquiring technology to manufacture the product will also impact on the choice of generic drug: • If the technology requires a fluidized bed coater, roller compactor, or any other special equipment, then the firm must consider whether this equipment i ...
Agricultural and Veterinary Chemicals Code Amendment
... bedding material, leather goods or surface coatings (including paint but excluding antifouling paint), if: (a) the mould inhibitor is incorporated into the product during manufacture for the protection of the goods; and (b) the mould inhibitor is not released into the environment from the manufactur ...
... bedding material, leather goods or surface coatings (including paint but excluding antifouling paint), if: (a) the mould inhibitor is incorporated into the product during manufacture for the protection of the goods; and (b) the mould inhibitor is not released into the environment from the manufactur ...
ANTI-INFLAMMATORY EFFECT OF THE SERRATIOPEPTIDASE
... chosen sub-therapeutic dose of diclofenac in combination group with the expectation to observe the anti-inflammatory properties of serratiopeptidase, if any. However, this study failed to show anti-inflammatory effects of serratiopeptidase alone or in combination. Diclofenac alone in therapeutic dos ...
... chosen sub-therapeutic dose of diclofenac in combination group with the expectation to observe the anti-inflammatory properties of serratiopeptidase, if any. However, this study failed to show anti-inflammatory effects of serratiopeptidase alone or in combination. Diclofenac alone in therapeutic dos ...
- Ontario.ca
... (7) Clauses (1) (c) and (h) do not apply to a product that is a solid oral dosage form for systemic effect and that has been designated by Health Canada as equivalent to the original product or to another listed interchangeable product with which it will be designated as interchangeable. O. Reg. 337 ...
... (7) Clauses (1) (c) and (h) do not apply to a product that is a solid oral dosage form for systemic effect and that has been designated by Health Canada as equivalent to the original product or to another listed interchangeable product with which it will be designated as interchangeable. O. Reg. 337 ...