Revised: April 2016 AN: 01396/2014 SUMMARY OF PRODUCT
... - very common (more than 1 in 10 animals displaying adverse reaction(s) during the course of one treatment - common (more than 1 but less than 10 animals in 100 animals) - uncommon (more than 1 but less than 10 animals in 1,000 animals) - rare (more than 1 but less than 10 animals in 10,000 animals) ...
... - very common (more than 1 in 10 animals displaying adverse reaction(s) during the course of one treatment - common (more than 1 but less than 10 animals in 100 animals) - uncommon (more than 1 but less than 10 animals in 1,000 animals) - rare (more than 1 but less than 10 animals in 10,000 animals) ...
Economic regulation of the pharmaceutical market (2)
... • Ukraine's accession to the PIC / S in 2011 and cancelation of laboratory control for medicines that are imported to Ukraine and are made by the companies situated on the territory of Member States PIC / S. • In 2011, the criminal liability for falsification of medicines was introduced. In the same ...
... • Ukraine's accession to the PIC / S in 2011 and cancelation of laboratory control for medicines that are imported to Ukraine and are made by the companies situated on the territory of Member States PIC / S. • In 2011, the criminal liability for falsification of medicines was introduced. In the same ...
Mucoangin oromucosal spray, solution SmPC
... Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions ...
... Reporting of suspected adverse reactions Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions ...
Slide 1
... assess the significance of potential drug-herb interactions, in particularly cardiovascular drugs – herbs interactions ...
... assess the significance of potential drug-herb interactions, in particularly cardiovascular drugs – herbs interactions ...
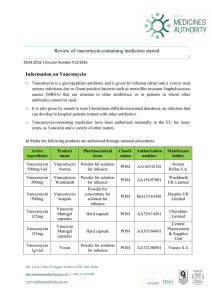
Review of vancomycin-containing medicines started Information on
... including vancomycin, it is considered of high relevance that the way this antibiotic is used in treating infections is re-assessed and that the product information for vancomycin-containing products is updated in light of available data. ...
... including vancomycin, it is considered of high relevance that the way this antibiotic is used in treating infections is re-assessed and that the product information for vancomycin-containing products is updated in light of available data. ...
Primovist solution for injection ENG SmPC
... according to System Organ Class (MedDRA version 12.1). The most appropriate MedDRA term is used to describe a certain reaction and its synonyms and related conditions. Adverse drug reactions from clinical trials are classified according to their frequencies. Frequency groupings are defined according ...
... according to System Organ Class (MedDRA version 12.1). The most appropriate MedDRA term is used to describe a certain reaction and its synonyms and related conditions. Adverse drug reactions from clinical trials are classified according to their frequencies. Frequency groupings are defined according ...
Simplifying NDA Programming with PROC SQL
... The programming of New Drug Application (NDA) Integrated Summary of Safety (ISS) usually involves obtaining patient counts, percentages, and other summary statistics such as mean, standard deviation and range. This paper shows how to obtain all of these results with the SQL procedure. While PROe SQL ...
... The programming of New Drug Application (NDA) Integrated Summary of Safety (ISS) usually involves obtaining patient counts, percentages, and other summary statistics such as mean, standard deviation and range. This paper shows how to obtain all of these results with the SQL procedure. While PROe SQL ...
New Drugs and Technologies
... the underlying mechanism of these events was common to all risk groups, the relative risks could be generalized. As seen in many recent cardiovascular outcome trials, despite enrolling a population at enhanced risk, the anticipated annual rate of cardiovascular events (projected to be 7%) was not ac ...
... the underlying mechanism of these events was common to all risk groups, the relative risks could be generalized. As seen in many recent cardiovascular outcome trials, despite enrolling a population at enhanced risk, the anticipated annual rate of cardiovascular events (projected to be 7%) was not ac ...
Bedside Teaching Trigger
... 2. The risk of a drug interaction greatly increases to approximately 50-60% when greater than 5 medications are taken. For patients taking 10 medications, there is approximately a 90% risk of drug interactions. 3. Approximately 50% of the elderly in the community setting take one or more unnecessary ...
... 2. The risk of a drug interaction greatly increases to approximately 50-60% when greater than 5 medications are taken. For patients taking 10 medications, there is approximately a 90% risk of drug interactions. 3. Approximately 50% of the elderly in the community setting take one or more unnecessary ...
Bedside Teaching Triggers
... 2. The risk of a drug interaction greatly increases to approximately 50-60% when greater than 5 medications are taken. For patients taking 10 medications, there is approximately a 90% risk of drug interactions. 3. Approximately 50% of the elderly in the community setting take one or more unnecessary ...
... 2. The risk of a drug interaction greatly increases to approximately 50-60% when greater than 5 medications are taken. For patients taking 10 medications, there is approximately a 90% risk of drug interactions. 3. Approximately 50% of the elderly in the community setting take one or more unnecessary ...
UKMi Benzodiazepine Dose Equivalents
... different half-life pre-discontinuation (4) or in the event of non-availability of a specific benzodiazepine. With relatively short-acting benzodiazepines such as alprazolam and lorazepam, it is not possible to achieve a smooth decline in blood and tissue concentrations during benzodiazepine withdra ...
... different half-life pre-discontinuation (4) or in the event of non-availability of a specific benzodiazepine. With relatively short-acting benzodiazepines such as alprazolam and lorazepam, it is not possible to achieve a smooth decline in blood and tissue concentrations during benzodiazepine withdra ...
ralbert
... period. Both IUDs have a four to five percent failure rate. Scientists are not certain how the IUD prevents pregnancy. Previously, researchers had reported that the IUD worked by making the uterus inhospitable to implantation. However, more recent evidence indicates that IUDs (particularly those con ...
... period. Both IUDs have a four to five percent failure rate. Scientists are not certain how the IUD prevents pregnancy. Previously, researchers had reported that the IUD worked by making the uterus inhospitable to implantation. However, more recent evidence indicates that IUDs (particularly those con ...
Slides
... • CMSC 2014 consensus statement says to initiate treatment with an FDA approved DMT • Availability of oral agents presents alternatives to injectable agents • Some suggest efficacy of the meds decreases if adherence is < 80% • Risks of certain adverse effects increase with comorbidities • The only d ...
... • CMSC 2014 consensus statement says to initiate treatment with an FDA approved DMT • Availability of oral agents presents alternatives to injectable agents • Some suggest efficacy of the meds decreases if adherence is < 80% • Risks of certain adverse effects increase with comorbidities • The only d ...
Medical Pharmacology 201 The Florida State University College of Medicine
... Relationship of course objectives to the “Six Principles” of the Curriculum: 1. The course is student-centered in providing a supportive, respectful environment in which to learn, while requiring that students be active and critical learners. 2. The course provides information that can be applied wi ...
... Relationship of course objectives to the “Six Principles” of the Curriculum: 1. The course is student-centered in providing a supportive, respectful environment in which to learn, while requiring that students be active and critical learners. 2. The course provides information that can be applied wi ...
Logistic Regression Part 2
... => equal health status, health behaviors => equal pre-clinical and clinical disease risk factors ...
... => equal health status, health behaviors => equal pre-clinical and clinical disease risk factors ...
Efficacy and safety of the biosimilar ABP 501 compared with
... inflammatory bowel disease, hidradenitis suppurativa and non-infectious intermediate and posterior uveitis and panuveitis; it is one of the most frequently prescribed biologics in clinical practice.2–6 Adalimumab has been extensively studied in combination with methotrexate (MTX) and has been shown ...
... inflammatory bowel disease, hidradenitis suppurativa and non-infectious intermediate and posterior uveitis and panuveitis; it is one of the most frequently prescribed biologics in clinical practice.2–6 Adalimumab has been extensively studied in combination with methotrexate (MTX) and has been shown ...
Parasite * Prolonged Activity - Veterinary Medicines Directorate
... No undesirable effects have been identified when the product is used at the recommended dose rate. 4.7 Use during pregnancy, lactation or lay May be used in dairy cattle during all stages of lactation. Studies have demonstrated a wide safety margin. Studies conducted at three times the recommended u ...
... No undesirable effects have been identified when the product is used at the recommended dose rate. 4.7 Use during pregnancy, lactation or lay May be used in dairy cattle during all stages of lactation. Studies have demonstrated a wide safety margin. Studies conducted at three times the recommended u ...
Successes, Threats, Challenges and Opportunities of Early
... Delivery of Candidate Drugs with reduced hERG liability R&D | Innovative Medicines | Global Safety Assessment ...
... Delivery of Candidate Drugs with reduced hERG liability R&D | Innovative Medicines | Global Safety Assessment ...
Redesign of a clinical decision support system for a drug - drug interaction alert
... Data Analysis: A descriptive cross-sectional study in which two types of units of analysis were considered was performed: the only positive combinations, by patient or episode, in the year of study, in order to determine their impact; and on the other hand, patients who met the inclusion criteria in ...
... Data Analysis: A descriptive cross-sectional study in which two types of units of analysis were considered was performed: the only positive combinations, by patient or episode, in the year of study, in order to determine their impact; and on the other hand, patients who met the inclusion criteria in ...
Concepts of Pharmacology - Half Life Calculation
... represent the time required for a drug to reach half of its initial concentration after administration • This is because in a single-compartment model elimination is the only process that can alter drug concentration • Intercompartmental distribution cannot occur because there are no other compartme ...
... represent the time required for a drug to reach half of its initial concentration after administration • This is because in a single-compartment model elimination is the only process that can alter drug concentration • Intercompartmental distribution cannot occur because there are no other compartme ...
File
... increase the cardiovascular risk associated with hypertension, there is no indication that smoking or alcohol ingestion are contributing to this patient's present problem. ...
... increase the cardiovascular risk associated with hypertension, there is no indication that smoking or alcohol ingestion are contributing to this patient's present problem. ...
Concepts of Pharmacology - Half Life Calculation -
... represent the time required for a drug to reach half of its initial concentration after administration • This is because in a single-compartment model elimination is the only process that can alter drug concentration • Intercompartmental distribution cannot occur because there are no other compartme ...
... represent the time required for a drug to reach half of its initial concentration after administration • This is because in a single-compartment model elimination is the only process that can alter drug concentration • Intercompartmental distribution cannot occur because there are no other compartme ...
NBER WORKING PAPER SERIES ASSESSING THE IMPACTS OF THE
... while FDA review times of the agency may have been fully met under PDUFA, the approval times are lagging considerably behind PDUFA goals on review times. The remainder of this paper proceeds as follows. In Section II we provide a brief introduction and overview of PDUFA-I and PDUFA-II. In Section II ...
... while FDA review times of the agency may have been fully met under PDUFA, the approval times are lagging considerably behind PDUFA goals on review times. The remainder of this paper proceeds as follows. In Section II we provide a brief introduction and overview of PDUFA-I and PDUFA-II. In Section II ...
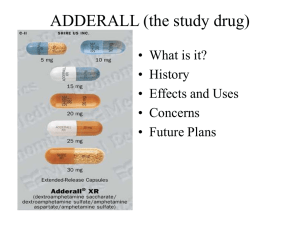
ADDERALL (the study drug)
... May cause existing mental illness's to worsen and possible psychosis; 10 or 11 recent cases Most were on adderall for longer than 3 years ...
... May cause existing mental illness's to worsen and possible psychosis; 10 or 11 recent cases Most were on adderall for longer than 3 years ...