< . 4- c
... Under 21 U.S. C. 350b(a), the manufacturer or distributor of a dietary supplement that contains a new dietary ingredient that has not been present in the food supply as an article used for food in a form in which the food has not been chemically altered must submit to FDA, at least 75 days before th ...
... Under 21 U.S. C. 350b(a), the manufacturer or distributor of a dietary supplement that contains a new dietary ingredient that has not been present in the food supply as an article used for food in a form in which the food has not been chemically altered must submit to FDA, at least 75 days before th ...
Patent Protection for Chinese Herbal Medicine Product Invention in
... purposes with no theories involved in their preparation. TCM or Kampo has been practiced in Japan since the fifth century (Department of Kampo Medicine, Keio University). As one of the first countries in the world to make it scientific, Japan has abolished all the TCM medical theories but retained just ...
... purposes with no theories involved in their preparation. TCM or Kampo has been practiced in Japan since the fifth century (Department of Kampo Medicine, Keio University). As one of the first countries in the world to make it scientific, Japan has abolished all the TCM medical theories but retained just ...
Complaint 1
... labeling may be cOr1lsidered a violation of the [Federal Food, Drug and Cosmetic] Act." ...
... labeling may be cOr1lsidered a violation of the [Federal Food, Drug and Cosmetic] Act." ...
Basics Pharmacology Review - Dr. Roland Halil
... Clinical Pharmacist, Bruyere Academic Family Health Team March 2014 rhalil@bruyere.org (Partially adapted from slides by Marc Riachi, R.Ph.) ...
... Clinical Pharmacist, Bruyere Academic Family Health Team March 2014 rhalil@bruyere.org (Partially adapted from slides by Marc Riachi, R.Ph.) ...
SNOMED CT® UK Drug Extension Editorial Policy
... Historical relationships relate inactive concepts to active concepts. For example, a concept may be inactivated because it is a duplicate. In this example a relationship is created when one concept is inactivated and stated to be the "same-as" another concept. Additional relationships are other non- ...
... Historical relationships relate inactive concepts to active concepts. For example, a concept may be inactivated because it is a duplicate. In this example a relationship is created when one concept is inactivated and stated to be the "same-as" another concept. Additional relationships are other non- ...
IOSR Journal of Pharmacy and Biological Sciences (IOSR-JPBS)
... cure was not yet discovered. Medications for treating immune responses are of main use for chronic arthritis patients but inflammat ion conditions can only be treated by NSAIDS, which g ives symptomatic relief. Though it will not cure the disease it will be a necessary adjunctive therapy for symptom ...
... cure was not yet discovered. Medications for treating immune responses are of main use for chronic arthritis patients but inflammat ion conditions can only be treated by NSAIDS, which g ives symptomatic relief. Though it will not cure the disease it will be a necessary adjunctive therapy for symptom ...
Glucosine 625 mg film-coated tablet ENG SmPC
... pharmacokinetic properties of glucosamine sulphate suggest a low potential for interactions. In addition glucosamine sulphate was found not to inhibit or to induce any of the major human CYP450 enzymes. In fact, the compound does not compete for absorption mechanisms and, after absorption, does not ...
... pharmacokinetic properties of glucosamine sulphate suggest a low potential for interactions. In addition glucosamine sulphate was found not to inhibit or to induce any of the major human CYP450 enzymes. In fact, the compound does not compete for absorption mechanisms and, after absorption, does not ...
1 - The University of Liverpool Repository
... initiated when an infected female Anopheles mosquito takes a blood meal during which infective forms of the parasite (named sporozoites) are injected into the blood stream (Figure 1).6 Within 15-30 minutes sporozoites reach the liver and infect hepatocytes where they develop into liver schizonts tha ...
... initiated when an infected female Anopheles mosquito takes a blood meal during which infective forms of the parasite (named sporozoites) are injected into the blood stream (Figure 1).6 Within 15-30 minutes sporozoites reach the liver and infect hepatocytes where they develop into liver schizonts tha ...
Pharmaceutical Administration and Regulations in Japan
... promotion of R&D, production, distribution policies, and drug pricing, i.e., functions related to pharmaceutical companies. The Pharmaceuticals and Medical Devices Evaluation Center (Evaluation Center) in the National Institute of Health Sciences was established to strengthen approval reviews on Jul ...
... promotion of R&D, production, distribution policies, and drug pricing, i.e., functions related to pharmaceutical companies. The Pharmaceuticals and Medical Devices Evaluation Center (Evaluation Center) in the National Institute of Health Sciences was established to strengthen approval reviews on Jul ...
THE KOREAN HERBAL PHARMACOPOEIA
... 7. In stating the amount of content in pharmaceutical preparations, the use of an expression “not less than 95.0% and not more than 105.0%” or “not less than 95.0% and not more than 110.0%”, for example, indicates that it is usually prepared so as to contain the labeled amount of the chemically pure ...
... 7. In stating the amount of content in pharmaceutical preparations, the use of an expression “not less than 95.0% and not more than 105.0%” or “not less than 95.0% and not more than 110.0%”, for example, indicates that it is usually prepared so as to contain the labeled amount of the chemically pure ...
How to Prevent Costly with QuickLabel Digital Label Printing Mislabeling and Recalls
... their products which were labeled incorrectly by their third-party manufacturer.1 In that situation, Pfizer’s Citalopram, a depression drug marketed as Celexa, was mislabeled as Finasteride, a drug used to treat cancer. And, in February 2011, Qualitest Pharmaceuticals voluntarily recalled Hydrocodon ...
... their products which were labeled incorrectly by their third-party manufacturer.1 In that situation, Pfizer’s Citalopram, a depression drug marketed as Celexa, was mislabeled as Finasteride, a drug used to treat cancer. And, in February 2011, Qualitest Pharmaceuticals voluntarily recalled Hydrocodon ...
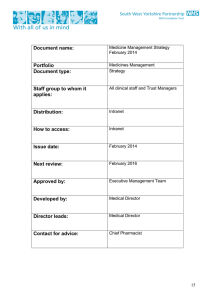
Medicines management strategy - South West Yorkshire Partnership
... Pharmacist. A performance report on incidents and activities is presented with performance monitoring every three months. Systems will therefore be established to monitor controlled drug prescribing which will be shared with intelligence networks to ensure safe and appropriate use of controlled drug ...
... Pharmacist. A performance report on incidents and activities is presented with performance monitoring every three months. Systems will therefore be established to monitor controlled drug prescribing which will be shared with intelligence networks to ensure safe and appropriate use of controlled drug ...
Assessment report - Voncento - HUMAN COAGULATION FACTOR
... The Drug Substance (S400 Bulk) is defined as Human Coagulation Factor FVIII/VWF Complex, purified and stabilised with albumin. The starting material for manufacturing human FVIII/VWF complex, human plasma, complies with the Ph. Eur. monograph 0853: “Human Plasma for Fractionation” and is described i ...
... The Drug Substance (S400 Bulk) is defined as Human Coagulation Factor FVIII/VWF Complex, purified and stabilised with albumin. The starting material for manufacturing human FVIII/VWF complex, human plasma, complies with the Ph. Eur. monograph 0853: “Human Plasma for Fractionation” and is described i ...
Prevalence and predictors of potential drug
... Abstract: Objectives. The objective of this study was to estimate the prevalence of potential drug-drug interactions (DDIs) among outpatients of city region Novi Sad, Serbia, and to investigate predictors of potential DDIs. Methods. Cross-sectional prescription database study was conducted. In the a ...
... Abstract: Objectives. The objective of this study was to estimate the prevalence of potential drug-drug interactions (DDIs) among outpatients of city region Novi Sad, Serbia, and to investigate predictors of potential DDIs. Methods. Cross-sectional prescription database study was conducted. In the a ...
Naturopathy Act, 2007 - O. Reg. 168/15
... member prescribed a drug for the patient and provide details respecting the prescription, unless the patient refuses to consent to the notification. 7. Where a limitation, a route of administration or a dosage is indicated in the column opposite the drug in Table 3, a member shall only prescribe tha ...
... member prescribed a drug for the patient and provide details respecting the prescription, unless the patient refuses to consent to the notification. 7. Where a limitation, a route of administration or a dosage is indicated in the column opposite the drug in Table 3, a member shall only prescribe tha ...
Biowaiver monographs for immediate release solid oral dosage forms
... Studies in humans in general show similar results. While most studies report no difference in extent of absorption, differences in rate of absorption between drug products were sometimes found. In one of the earliest relevant studies, Sotiropoulus et al.[69] evaluated three tablets and one liquid ac ...
... Studies in humans in general show similar results. While most studies report no difference in extent of absorption, differences in rate of absorption between drug products were sometimes found. In one of the earliest relevant studies, Sotiropoulus et al.[69] evaluated three tablets and one liquid ac ...
Naturopathy Act, 2007 - O. Reg. 168/15
... member prescribed a drug for the patient and provide details respecting the prescription, unless the patient refuses to consent to the notification. 7. Where a limitation, a route of administration or a dosage is indicated in the column opposite the drug in Table 3, a member shall only prescribe tha ...
... member prescribed a drug for the patient and provide details respecting the prescription, unless the patient refuses to consent to the notification. 7. Where a limitation, a route of administration or a dosage is indicated in the column opposite the drug in Table 3, a member shall only prescribe tha ...
Metabolism and drug interactions of 3-hydroxy-3
... Concerning drug interactions, the metabolism by cytochrome P450 enzymes (CYP) seems to be the most important [19, 20, 21]. These enzymes are expressed mainly in liver microsomes and in gut wall [22]. The CYP3A iso-enzymes are the most abundant and account for approximately 30% in liver and 80% in sm ...
... Concerning drug interactions, the metabolism by cytochrome P450 enzymes (CYP) seems to be the most important [19, 20, 21]. These enzymes are expressed mainly in liver microsomes and in gut wall [22]. The CYP3A iso-enzymes are the most abundant and account for approximately 30% in liver and 80% in sm ...
10.4103-0975-1483.93570
... 8.0 g of sodium hydroxide (NaOH) was dissolved and diluted to 1000 ml with deminerali ed water.[9] Preparation of phosphate buffer (pH=6.8): 250 ml of mono basic potassium phosphate (KH2PO4) was placed in a 1000 ml volumetric ask, 112 ml of 0.2M NaOH was added and volume was made up to 1000 ml with ...
... 8.0 g of sodium hydroxide (NaOH) was dissolved and diluted to 1000 ml with deminerali ed water.[9] Preparation of phosphate buffer (pH=6.8): 250 ml of mono basic potassium phosphate (KH2PO4) was placed in a 1000 ml volumetric ask, 112 ml of 0.2M NaOH was added and volume was made up to 1000 ml with ...
Development of Discriminative Dissolution Media for Marketed
... Based on this, in vitro dissolution may be vital in assessing in vivo performance. Dissolution testing serves as a tool to distinguish between acceptable and unacceptable products. It is also used to assess the lot-to-lot quality of a drug product and can guide the development of new formulations. I ...
... Based on this, in vitro dissolution may be vital in assessing in vivo performance. Dissolution testing serves as a tool to distinguish between acceptable and unacceptable products. It is also used to assess the lot-to-lot quality of a drug product and can guide the development of new formulations. I ...