Biopharmaceutics Clasification System (BCS)
... The major challenge in development of drug delivery system for class I drugs is to achieve a target release profile associated with a particular pharmcokinetic and/or pharmacodynamic profile. Formulation approaches include both control of release rate and certain physicochemical properties of drugs ...
... The major challenge in development of drug delivery system for class I drugs is to achieve a target release profile associated with a particular pharmcokinetic and/or pharmacodynamic profile. Formulation approaches include both control of release rate and certain physicochemical properties of drugs ...
DSG - TMA
... Based on a solid, scientifically sound platform for data collection and processing Tobacco Merchants Association, Williamsburg, VA ...
... Based on a solid, scientifically sound platform for data collection and processing Tobacco Merchants Association, Williamsburg, VA ...
ACORDA THERAPEUTICS INC (Form: 8-K
... In addition, testing detected rHIgM22 in cerebrospinal fluid (CSF), indicating the drug’s access to the central nervous system. These data were presented at the 67 th American Academy of Neurology Annual Meeting in Washington, DC. “In this study, rHIgM22 was well-tolerated over the full range of dos ...
... In addition, testing detected rHIgM22 in cerebrospinal fluid (CSF), indicating the drug’s access to the central nervous system. These data were presented at the 67 th American Academy of Neurology Annual Meeting in Washington, DC. “In this study, rHIgM22 was well-tolerated over the full range of dos ...
Dr. Mary Teeling Dept. of Pharmacology & Therapeutics / Centre for
... women who received stilboestrol during pregnancy – 20 year time lapse **Not predictable, often hard to detect but pharmacovigilance may pick them up eventually** ...
... women who received stilboestrol during pregnancy – 20 year time lapse **Not predictable, often hard to detect but pharmacovigilance may pick them up eventually** ...
Nebilet (Chronic Heart Failure) - Forecast and Market Analysis to... Brochure
... The chronic heart failure (CHF) market is a mature market that has been slowly overtaken by generic drugs, and more branded products are expected to lose market exclusivity during the next few years. GlobalData predicts that the major global barriers that will play a crucial role in narrowing the gl ...
... The chronic heart failure (CHF) market is a mature market that has been slowly overtaken by generic drugs, and more branded products are expected to lose market exclusivity during the next few years. GlobalData predicts that the major global barriers that will play a crucial role in narrowing the gl ...
TGA to cancel ALL dextropropoxyphene products The TGA
... post-operative and post-partum settings, the 3 months advance notice will allow prescribers time to transition their prescribing practices to alternative analgesics. To read the TGA announcement, click here. Withdrawal of dextropropoxyphene-containing analgesics from the market brings Australia in l ...
... post-operative and post-partum settings, the 3 months advance notice will allow prescribers time to transition their prescribing practices to alternative analgesics. To read the TGA announcement, click here. Withdrawal of dextropropoxyphene-containing analgesics from the market brings Australia in l ...
Breastfeeding and Medications - Central MN Breastfeeding Coalition
... New drugs – not studied in these patients Risks change during breastfeeding Neonate and very young at most risk Nearly all reported adverse effects have occurred in infants < 6 months old Recommendations based on toxicity data for adults in ...
... New drugs – not studied in these patients Risks change during breastfeeding Neonate and very young at most risk Nearly all reported adverse effects have occurred in infants < 6 months old Recommendations based on toxicity data for adults in ...
Research and Development at Sun Pharma
... At equivalent dosage, AUC 1.6 times higher than existing marketed products Substantially improved efficacy seen in muscle coordination in animal model Current global market size : USD 200 million (all existing products are generic) No. of spastic patients under treatment (US+Europe) : 1.5 mil ...
... At equivalent dosage, AUC 1.6 times higher than existing marketed products Substantially improved efficacy seen in muscle coordination in animal model Current global market size : USD 200 million (all existing products are generic) No. of spastic patients under treatment (US+Europe) : 1.5 mil ...
Research and Development at Sun Pharma
... At equivalent dosage, AUC 1.6 times higher than existing marketed products Substantially improved efficacy seen in muscle coordination in animal model Current global market size : USD 200 million (all existing products are generic) No. of spastic patients under treatment (US+Europe) : 1.5 mil ...
... At equivalent dosage, AUC 1.6 times higher than existing marketed products Substantially improved efficacy seen in muscle coordination in animal model Current global market size : USD 200 million (all existing products are generic) No. of spastic patients under treatment (US+Europe) : 1.5 mil ...
Integration with robot drug dispenser
... ‘Increased patient safety was the objective when we started using the robot drug dispenser, and as expected there have not been any errors with the automatic drug preparation process. There are also clearly going to be cost savings – from nurses spending less time having to measure out patients’ med ...
... ‘Increased patient safety was the objective when we started using the robot drug dispenser, and as expected there have not been any errors with the automatic drug preparation process. There are also clearly going to be cost savings – from nurses spending less time having to measure out patients’ med ...
Full DNA Polymerase Enzyme Mix
... Clontech products are to be used for research purposes only. They may not be used for any other purpose, including, but not limited to, use in drugs, in vitro diagnostic purposes, therapeutics, or in humans. Clontech products may not be transferred to third parties, resold, modified for resale, or u ...
... Clontech products are to be used for research purposes only. They may not be used for any other purpose, including, but not limited to, use in drugs, in vitro diagnostic purposes, therapeutics, or in humans. Clontech products may not be transferred to third parties, resold, modified for resale, or u ...
Document
... Portion of PPCPs in environment originating from disposal versus excretion is not known. Public identifies strongly with the topic and is concerned about the possibility for residues in drinking water. Inquiries continually received from public, media, healthcare community, and regulators regarding ...
... Portion of PPCPs in environment originating from disposal versus excretion is not known. Public identifies strongly with the topic and is concerned about the possibility for residues in drinking water. Inquiries continually received from public, media, healthcare community, and regulators regarding ...
Reduction in Medication Errors: The Fentora Case Study
... A safety alert issued on September 13, 2007, stated that health care professionals should strictly abide by the labeling information in the approved labeling and not prescribe Fentora for minor pains, such as headache or regular pain.13 FDA issued another safety alert in January 2008 stating that Fe ...
... A safety alert issued on September 13, 2007, stated that health care professionals should strictly abide by the labeling information in the approved labeling and not prescribe Fentora for minor pains, such as headache or regular pain.13 FDA issued another safety alert in January 2008 stating that Fe ...
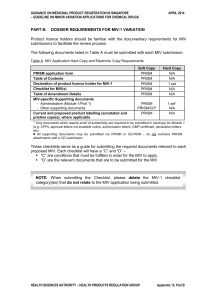
part b: dossier requirements for miv-1 variation
... variation (where applicable). 2. Proof that the proposed site is appropriately authorised for the pharmaceutical form concerned, such as a valid Good Manufacturing Practice (GMP) certificate and/or a Certificate of Pharmaceutical Product (CPP) which covers GMP certification. (Note: GMP Conformity As ...
... variation (where applicable). 2. Proof that the proposed site is appropriately authorised for the pharmaceutical form concerned, such as a valid Good Manufacturing Practice (GMP) certificate and/or a Certificate of Pharmaceutical Product (CPP) which covers GMP certification. (Note: GMP Conformity As ...
Patients Perspective Towards Mail Order Services
... • FDA approved as adjunct to diet and exercise to control blood glucose. • Also studied in combination with metformin, SU, insulin, pioglitazone • Can be used as second line, after metformin ( because metformin is more studied and approved as first line), however, its cost should be considered. ...
... • FDA approved as adjunct to diet and exercise to control blood glucose. • Also studied in combination with metformin, SU, insulin, pioglitazone • Can be used as second line, after metformin ( because metformin is more studied and approved as first line), however, its cost should be considered. ...
Uniseed Overview and Investments November 2014 1
... Fibrotech is developing drugs that target the fibrosis of kidney and other disorders. ...
... Fibrotech is developing drugs that target the fibrosis of kidney and other disorders. ...