Nuclear Fission vs Fusion
... (Spl“i”tting an atom into two new ones) In nuclear fission reactions (also called radioactive decay), a neutron is aimed at the nucleus of a large, unstable atom, like uranium, thorium, or other radioactive elements. The extra mass of the neutron causes the radioactive nucleus to split apart, formin ...
... (Spl“i”tting an atom into two new ones) In nuclear fission reactions (also called radioactive decay), a neutron is aimed at the nucleus of a large, unstable atom, like uranium, thorium, or other radioactive elements. The extra mass of the neutron causes the radioactive nucleus to split apart, formin ...
Nuclear Fission vs. Nuclear Fusion
... (Spl“i”tting an atom into two new ones) In nuclear fission reactions (also called radioactive decay), a neutron is aimed at the nucleus of a large, unstable atom, like uranium, thorium, or other radioactive elements. The extra mass of the neutron causes the radioactive nucleus to split apart, formin ...
... (Spl“i”tting an atom into two new ones) In nuclear fission reactions (also called radioactive decay), a neutron is aimed at the nucleus of a large, unstable atom, like uranium, thorium, or other radioactive elements. The extra mass of the neutron causes the radioactive nucleus to split apart, formin ...
Nuclear Chemistry Test Topics
... Nuclear fission occurs when an unstable nucleus is bombarded by a neutron. This collision causes the nucleus to break apart and release large amounts of energy. The pieces of the broken nucleus bombard other unstable nuclei in the sample of material causing them to break apart. This chain of one nuc ...
... Nuclear fission occurs when an unstable nucleus is bombarded by a neutron. This collision causes the nucleus to break apart and release large amounts of energy. The pieces of the broken nucleus bombard other unstable nuclei in the sample of material causing them to break apart. This chain of one nuc ...
Unit 14 Notes - shscience.net
... of another element can occur by radioactive decay (natural) or when particles bombard the nucleus of an atom (artificial) ...
... of another element can occur by radioactive decay (natural) or when particles bombard the nucleus of an atom (artificial) ...
Nuclear Chemistry - Xavier High School
... • Nuclear reactions involve the nucleus • The nucleus opens, and protons and neutrons are rearranged • The opening of the nucleus releases a tremendous amount of energy that holds the nucleus together – called binding energy • “Normal” Chemical Reactions involve electrons, not protons and neutrons ...
... • Nuclear reactions involve the nucleus • The nucleus opens, and protons and neutrons are rearranged • The opening of the nucleus releases a tremendous amount of energy that holds the nucleus together – called binding energy • “Normal” Chemical Reactions involve electrons, not protons and neutrons ...
Atom and Nucleus. Radioactivity. Nuclear Energy.
... A.Becquerel found that uranium (U) exposed photographic film. In other words, uranium emitted penetrating radiation. Marie and Pierre Curie soon discovered 2 more radioactive elements, which were called polonium and radium. Radioactivity is associated with the nucleus and is not affected by chemical ...
... A.Becquerel found that uranium (U) exposed photographic film. In other words, uranium emitted penetrating radiation. Marie and Pierre Curie soon discovered 2 more radioactive elements, which were called polonium and radium. Radioactivity is associated with the nucleus and is not affected by chemical ...
Nuclear Chemistry
... nuclear forces that overcome the electromagnetic repulsion between the protons. b. Students know the energy release per gram of material is much larger in nuclear fusion or fission reactions than in chemical reactions. The change in mass (calculated by E = mc2) is small but significant in nuclear re ...
... nuclear forces that overcome the electromagnetic repulsion between the protons. b. Students know the energy release per gram of material is much larger in nuclear fusion or fission reactions than in chemical reactions. The change in mass (calculated by E = mc2) is small but significant in nuclear re ...
Unit 2 – The Atom
... Fission Chain Reactions • One fission reaction can lead to more fission reactions in a process called a chain reaction. • Example - The fission of Uranium-235 ...
... Fission Chain Reactions • One fission reaction can lead to more fission reactions in a process called a chain reaction. • Example - The fission of Uranium-235 ...
Nuclear Stability
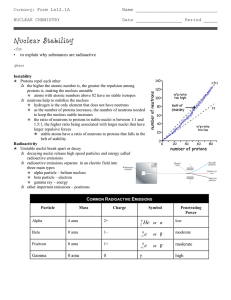
... • All nuclides with 84 or more protons are unstable with respect to radio active decay. • Light nuclides are stable when neutron/proton = 1. For heavier elements the neutron /proton ratio required for stability is greater than 1 and increases with Z. • Nuclides with even numbers of protons and neutr ...
... • All nuclides with 84 or more protons are unstable with respect to radio active decay. • Light nuclides are stable when neutron/proton = 1. For heavier elements the neutron /proton ratio required for stability is greater than 1 and increases with Z. • Nuclides with even numbers of protons and neutr ...
Radioactivity - Mrs. Sjuts` Science Site
... Chain reaction – ongoing series of fission reactions Critical mass – the amount of fissionable material required so that each fission reaction produce approximately one more fission reaction ...
... Chain reaction – ongoing series of fission reactions Critical mass – the amount of fissionable material required so that each fission reaction produce approximately one more fission reaction ...
Serway_PSE_quick_ch45
... have too many neutrons for the nucleus to be stable. Beta decay in which electrons are ejected decreases the number of neutrons and increases the number of protons in order to stabilize the nucleus. ...
... have too many neutrons for the nucleus to be stable. Beta decay in which electrons are ejected decreases the number of neutrons and increases the number of protons in order to stabilize the nucleus. ...
Radioactivity
... Radioactivity is the spontaneous disintegration (= decay) of the nucleus of an atom, from which may be emitted some or all of the followings: 1/ -particle 2/ -particle 3/ rays This spontaneous process is not affected by: a/ chemical combination (e.g. ________________) b/ ...
... Radioactivity is the spontaneous disintegration (= decay) of the nucleus of an atom, from which may be emitted some or all of the followings: 1/ -particle 2/ -particle 3/ rays This spontaneous process is not affected by: a/ chemical combination (e.g. ________________) b/ ...
Fission and Fusion Power Point
... In this example, a stray neutron strikes an atom of U-235. It absorbs the neutron and becomes an unstable atom of U-236. It then undergoes fission and splits into two daughter nuclides. ...
... In this example, a stray neutron strikes an atom of U-235. It absorbs the neutron and becomes an unstable atom of U-236. It then undergoes fission and splits into two daughter nuclides. ...
Review of Nuclear Chemistry
... You should know how penetrating and the relative speeds of the particles in Table 1. An alpha particle is the largest radiation particle; while a gamma particle is the fastest. A gamma particle is the most dangerous form of radiation outside the body, while an alpha particle is the most dangerous in ...
... You should know how penetrating and the relative speeds of the particles in Table 1. An alpha particle is the largest radiation particle; while a gamma particle is the fastest. A gamma particle is the most dangerous form of radiation outside the body, while an alpha particle is the most dangerous in ...
File
... Gamma Decay Gamma rays are not charged particles like a and b particles. They are electromagnetic radiation with very high energy When atoms decay by emitting a or b particles to form a new atom, the nuclei of the new atom formed may still have too much energy to be completely stable. ...
... Gamma Decay Gamma rays are not charged particles like a and b particles. They are electromagnetic radiation with very high energy When atoms decay by emitting a or b particles to form a new atom, the nuclei of the new atom formed may still have too much energy to be completely stable. ...
Terms to Know
... Beta particles : A beta particle is an electron emitted by the nucleus of a radioactive atom. Decomposition : A chemical reaction in which a compound is broken down into simpler compounds or elements. Fission : When a neutron strikes the nucleus of certain isotopes, the nucleus breaks or splits apar ...
... Beta particles : A beta particle is an electron emitted by the nucleus of a radioactive atom. Decomposition : A chemical reaction in which a compound is broken down into simpler compounds or elements. Fission : When a neutron strikes the nucleus of certain isotopes, the nucleus breaks or splits apar ...
Nuclear Fission sim
... the same. Carbon-14 (atomic number is 6) to Nitrogen (atomic number is 7). ...
... the same. Carbon-14 (atomic number is 6) to Nitrogen (atomic number is 7). ...
Fission and Fusion
... In this example, a stray neutron strikes an atom of U-235. It absorbs the neutron and becomes an unstable atom of U-236. It then undergoes fission. Notice that more neutrons are released in the reaction. These neutrons can strike other U-235 atoms to initiate their fission. ...
... In this example, a stray neutron strikes an atom of U-235. It absorbs the neutron and becomes an unstable atom of U-236. It then undergoes fission. Notice that more neutrons are released in the reaction. These neutrons can strike other U-235 atoms to initiate their fission. ...
Nuclear Stability
... q the higher the atomic number is, the greater the repulsion among protons is, making the nucleus unstable p atoms with atomic numbers above 82 have no stable isotopes q neutrons help to stabilize the nucleus p hydrogen is the only element that does not have neutrons p as the number of protons incre ...
... q the higher the atomic number is, the greater the repulsion among protons is, making the nucleus unstable p atoms with atomic numbers above 82 have no stable isotopes q neutrons help to stabilize the nucleus p hydrogen is the only element that does not have neutrons p as the number of protons incre ...
Chapter 19 Nuclear Chemistry
... • The energy required to decompose the nucleus into its components. • Iron-56 is the most stable nucleus and has a binding energy of 8.97 MeV. ...
... • The energy required to decompose the nucleus into its components. • Iron-56 is the most stable nucleus and has a binding energy of 8.97 MeV. ...
Fission vs Fusion Worksheet
... fission reactions similar to those in nuclear reactors [power plants], and hydrogen bombs, which derive their explosive power from fusion reactions. An atomic bomb slams together two pieces of fissionable material, usually uranium-235 or plutonium-239. This releases its energy instantaneously as ato ...
... fission reactions similar to those in nuclear reactors [power plants], and hydrogen bombs, which derive their explosive power from fusion reactions. An atomic bomb slams together two pieces of fissionable material, usually uranium-235 or plutonium-239. This releases its energy instantaneously as ato ...
What do I know about……
... describe Rutherford’s nuclear model of the atom and how it accounts for the results of Geiger and Marsden’s experiment and understand the factors (charge and speed) which affect the deflection of alpha particles by a nucleus understand that a nucleus of U-235 can be split (the process of fission) by ...
... describe Rutherford’s nuclear model of the atom and how it accounts for the results of Geiger and Marsden’s experiment and understand the factors (charge and speed) which affect the deflection of alpha particles by a nucleus understand that a nucleus of U-235 can be split (the process of fission) by ...
Nuclear Power Date
... 13. What is one benefit associated with a nuclear fission reaction? 1) The products are not radioactive. 2) Stable isotopes are used as reactants. 3) There is no chance of biological exposure. 4) A large amount of energy is produced. 14. Given the balanced equation representing a nuclear reaction: ...
... 13. What is one benefit associated with a nuclear fission reaction? 1) The products are not radioactive. 2) Stable isotopes are used as reactants. 3) There is no chance of biological exposure. 4) A large amount of energy is produced. 14. Given the balanced equation representing a nuclear reaction: ...
Nuclear fission
In nuclear physics and nuclear chemistry, nuclear fission is either a nuclear reaction or a radioactive decay process in which the nucleus of an atom splits into smaller parts (lighter nuclei). The fission process often produces free neutrons and photons (in the form of gamma rays), and releases a very large amount of energy even by the energetic standards of radioactive decay.Nuclear fission of heavy elements was discovered on December 17, 1938 by German Otto Hahn and his assistant Fritz Strassmann, and explained theoretically in January 1939 by Lise Meitner and her nephew Otto Robert Frisch. Frisch named the process by analogy with biological fission of living cells. It is an exothermic reaction which can release large amounts of energy both as electromagnetic radiation and as kinetic energy of the fragments (heating the bulk material where fission takes place). In order for fission to produce energy, the total binding energy of the resulting elements must be less negative (higher energy) than that of the starting element.Fission is a form of nuclear transmutation because the resulting fragments are not the same element as the original atom. The two nuclei produced are most often of comparable but slightly different sizes, typically with a mass ratio of products of about 3 to 2, for common fissile isotopes. Most fissions are binary fissions (producing two charged fragments), but occasionally (2 to 4 times per 1000 events), three positively charged fragments are produced, in a ternary fission. The smallest of these fragments in ternary processes ranges in size from a proton to an argon nucleus.Apart from fission induced by a neutron, harnessed and exploited by humans, a natural form of spontaneous radioactive decay (not requiring a neutron) is also referred to as fission, and occurs especially in very high-mass-number isotopes. Spontaneous fission was discovered in 1940 by Flyorov, Petrzhak and Kurchatov in Moscow, when they decided to confirm that, without bombardment by neutrons, the fission rate of uranium was indeed negligible, as predicted by Niels Bohr; it wasn't.The unpredictable composition of the products (which vary in a broad probabilistic and somewhat chaotic manner) distinguishes fission from purely quantum-tunnelling processes such as proton emission, alpha decay and cluster decay, which give the same products each time. Nuclear fission produces energy for nuclear power and drives the explosion of nuclear weapons. Both uses are possible because certain substances called nuclear fuels undergo fission when struck by fission neutrons, and in turn emit neutrons when they break apart. This makes possible a self-sustaining nuclear chain reaction that releases energy at a controlled rate in a nuclear reactor or at a very rapid uncontrolled rate in a nuclear weapon.The amount of free energy contained in nuclear fuel is millions of times the amount of free energy contained in a similar mass of chemical fuel such as gasoline, making nuclear fission a very dense source of energy. The products of nuclear fission, however, are on average far more radioactive than the heavy elements which are normally fissioned as fuel, and remain so for significant amounts of time, giving rise to a nuclear waste problem. Concerns over nuclear waste accumulation and over the destructive potential of nuclear weapons may counterbalance the desirable qualities of fission as an energy source, and give rise to ongoing political debate over nuclear power.