Stoichiometry and the Mole
... College of Education. The 100,000-square-foot building has enough office space to accommodate 86 full-time faculty members and 167 full-time staff. In a fit of monetary excess, the university administration offered to buy new furniture (desks and chairs) and computer workstations for all faculty and staff ...
... College of Education. The 100,000-square-foot building has enough office space to accommodate 86 full-time faculty members and 167 full-time staff. In a fit of monetary excess, the university administration offered to buy new furniture (desks and chairs) and computer workstations for all faculty and staff ...
Chapter Three
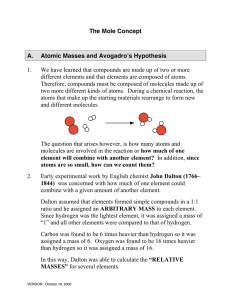
... sodium, 22.67% sulfur, and 45.02% oxygen. Find the empirical formula for this compound. • HINT = Whenever you are given %s, we assume we ALWAYS have a 100-gram sample, so immediately convert you %s to grams. ...
... sodium, 22.67% sulfur, and 45.02% oxygen. Find the empirical formula for this compound. • HINT = Whenever you are given %s, we assume we ALWAYS have a 100-gram sample, so immediately convert you %s to grams. ...
Experimental Study of Closed System in the Chlorine Dioxide
... When the mole ratio r is below or equal to 1.00 (see curves 1 and 2), the absorbance decreases along with the extension of reaction time at 350 nm and then does not change with the reaction time afterwards. Under the condition that r is greater than 1.00 (see curve 3 to curve 7), the absorbance incr ...
... When the mole ratio r is below or equal to 1.00 (see curves 1 and 2), the absorbance decreases along with the extension of reaction time at 350 nm and then does not change with the reaction time afterwards. Under the condition that r is greater than 1.00 (see curve 3 to curve 7), the absorbance incr ...
Chapter 4 Chemical Quantities and Aqueous Reactions
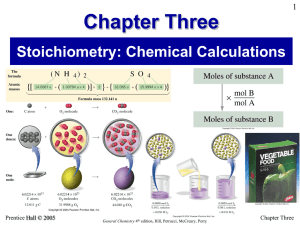
... 2 molecules of C8H18 react with 25 molecules of O2 to form 16 molecules of CO2 and 18 molecules of H2O 2 moles of C8H18 react with 25 moles of O2 to form 16 moles of CO2 and 18 moles of H2O 2 mol C8H18 : 25 mol O2 : 16 mol CO2 : 18 mol H2O ...
... 2 molecules of C8H18 react with 25 molecules of O2 to form 16 molecules of CO2 and 18 molecules of H2O 2 moles of C8H18 react with 25 moles of O2 to form 16 moles of CO2 and 18 moles of H2O 2 mol C8H18 : 25 mol O2 : 16 mol CO2 : 18 mol H2O ...
Document
... – Remember that this carbon originally from the 0.1156‐gram sample of the unknown compound. Thus the mass percent of carbon in this compound is (0.04470 g C/0.1156 g compound) 100% = 38.67% C ...
... – Remember that this carbon originally from the 0.1156‐gram sample of the unknown compound. Thus the mass percent of carbon in this compound is (0.04470 g C/0.1156 g compound) 100% = 38.67% C ...
Novel Class of Heterometallic Cubane and Boride Clusters
... interstitial borides has produced a richly developed area of cluster chemistry.32 Metallaboranes are predominantly exemplified by boron-rich clusters rather than metal-rich clusters.33−39 The characteristic feature that separates the boride clusters40 from the metallaboranes is the greater number of ...
... interstitial borides has produced a richly developed area of cluster chemistry.32 Metallaboranes are predominantly exemplified by boron-rich clusters rather than metal-rich clusters.33−39 The characteristic feature that separates the boride clusters40 from the metallaboranes is the greater number of ...
CYPRUS
... bibliographic studies and problem-solving sessions. Chemistry is, however, by nature an experimental science. For this reason, the curriculum of the Department places strong emphasis on laboratory courses, which are regarded as independent courses, and not as complements to existing theoretical cour ...
... bibliographic studies and problem-solving sessions. Chemistry is, however, by nature an experimental science. For this reason, the curriculum of the Department places strong emphasis on laboratory courses, which are regarded as independent courses, and not as complements to existing theoretical cour ...
CHEMISTRY OF p-ELEMENTS - Львівський національний
... including proteins, nucleic acids, hydrocarbons, enzymes, vitamins. The study of life is known as biological chemistry or biochemistry. Oxygen atoms are present in water (H2O) and water is essential to all life. Oxygen is present in many organic compounds. Most organisms use oxygen for respiration. ...
... including proteins, nucleic acids, hydrocarbons, enzymes, vitamins. The study of life is known as biological chemistry or biochemistry. Oxygen atoms are present in water (H2O) and water is essential to all life. Oxygen is present in many organic compounds. Most organisms use oxygen for respiration. ...
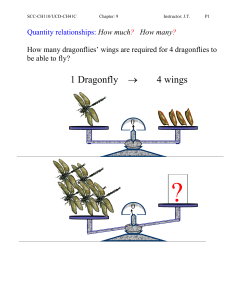
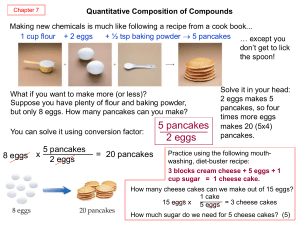
Quantity relationships: How much
... The calculated amount of product if is based on the assumption that all of the reactant is converted into product is called the theoretical yield. In laboratory or in industrial production, the actual amount of product isolated from a reaction is usually less than the theoretical yield, and it is ca ...
... The calculated amount of product if is based on the assumption that all of the reactant is converted into product is called the theoretical yield. In laboratory or in industrial production, the actual amount of product isolated from a reaction is usually less than the theoretical yield, and it is ca ...
1 mol H 2
... amounts of reactants used and the products formed by a chemical reaction. It is based on the Law of Conservation of Mass: the amount of matter present at the end of a reaction is the same as was present at the beginning. The total mass of the reactants equals the mass of the products. ...
... amounts of reactants used and the products formed by a chemical reaction. It is based on the Law of Conservation of Mass: the amount of matter present at the end of a reaction is the same as was present at the beginning. The total mass of the reactants equals the mass of the products. ...
General chemistry laboratory activities, Lorentz
... Reaction flasks (see Figure 2) are usually spherical (i.e. round-bottom flask) and are accompanied by their necks, at the ends of which are ground glass joints to quickly and tightly connect to the rest of the apparatus (such as a reflux condenser or dropping funnel). The reaction flask is usually m ...
... Reaction flasks (see Figure 2) are usually spherical (i.e. round-bottom flask) and are accompanied by their necks, at the ends of which are ground glass joints to quickly and tightly connect to the rest of the apparatus (such as a reflux condenser or dropping funnel). The reaction flask is usually m ...
word - My eCoach
... A. It has a mass of 116 g. B. It has a mass of 164 amu. C. It contains 6 oxygen atoms. D. It contains 1.204 x 1024 nitrate (NO3-) ions. 10) 9.03 x 1023 atoms of silver are placed on a balance. The balance should read A. 53.96 g B. 107.87g C. 161.81g D. 215.74g Part 2. Free Response. Answer the quest ...
... A. It has a mass of 116 g. B. It has a mass of 164 amu. C. It contains 6 oxygen atoms. D. It contains 1.204 x 1024 nitrate (NO3-) ions. 10) 9.03 x 1023 atoms of silver are placed on a balance. The balance should read A. 53.96 g B. 107.87g C. 161.81g D. 215.74g Part 2. Free Response. Answer the quest ...
technical report 91 -32
... radioactive elements to the biosphere. Intrusion of water into the repository could lead to leaching of the radioactive elements and migration through the barriers into surface waters from where they could enter the food chain. Government regulations set a limit for radiation doses resulting from su ...
... radioactive elements to the biosphere. Intrusion of water into the repository could lead to leaching of the radioactive elements and migration through the barriers into surface waters from where they could enter the food chain. Government regulations set a limit for radiation doses resulting from su ...
ION-SELECTIVE ELECTRODES - Clayton State University
... - The study of the interconversion of chemical energy and electrical energy - The study of redox reactions - Electrochemical processes involve the transfer of electrons from one substance to another ...
... - The study of the interconversion of chemical energy and electrical energy - The study of redox reactions - Electrochemical processes involve the transfer of electrons from one substance to another ...
Document
... Suppose you want to ‘whip’ a batch of hydrogen iodide, following the balanced chemical equation: ...
... Suppose you want to ‘whip’ a batch of hydrogen iodide, following the balanced chemical equation: ...
chemistry - Brilliant Public School Sitamarhi
... *13. The density of copper metal is 8.95 g cm–3. If the radius of copper atom is 127 pm, is the copper unit cell a simple cubic, a body-centred cubic or a face centred cubic structure? (Given at. mass of Cu = 63.54 g mol–1 and NA = 6.02 × 1023 mol–1] [Ans. : Z = 4, fcc type] [Hint : d = ...
... *13. The density of copper metal is 8.95 g cm–3. If the radius of copper atom is 127 pm, is the copper unit cell a simple cubic, a body-centred cubic or a face centred cubic structure? (Given at. mass of Cu = 63.54 g mol–1 and NA = 6.02 × 1023 mol–1] [Ans. : Z = 4, fcc type] [Hint : d = ...
Under Choice Based Credit System Proposed syllabus and Scheme of Examination
... and angular parts of the hydogenic wavefunctions (atomic orbitals) and their variations for 1s, 2s, 2p, 3s, 3p and 3d orbitals (Only graphical representation). Radial and angular nodes and their significance. Radial distribution functions and the concept of the most probable distance with special re ...
... and angular parts of the hydogenic wavefunctions (atomic orbitals) and their variations for 1s, 2s, 2p, 3s, 3p and 3d orbitals (Only graphical representation). Radial and angular nodes and their significance. Radial distribution functions and the concept of the most probable distance with special re ...
PHOSPHORUS AND SULFUR COSMOCHEMISTRY
... phosphorus compounds, energetic organic compounds, or unusual physical conditions. Meteoritic schreibersite provided an abundant source of reactive phosphorus for the early Earth. Water corrodes schreibersite to form a mixed valence series of phosphorus compounds. Schreibersite corrosion was studied ...
... phosphorus compounds, energetic organic compounds, or unusual physical conditions. Meteoritic schreibersite provided an abundant source of reactive phosphorus for the early Earth. Water corrodes schreibersite to form a mixed valence series of phosphorus compounds. Schreibersite corrosion was studied ...
Complete Solution Manual
... The zero point for standard reduction potentials (E) is the standard hydrogen electrode. The half-reaction is: 2 H+ + 2 e → H2. This half-reaction is assigned a standard potential of zero, and all other reduction half-reactions are measured relative to this zero point. Substances less easily reduc ...
... The zero point for standard reduction potentials (E) is the standard hydrogen electrode. The half-reaction is: 2 H+ + 2 e → H2. This half-reaction is assigned a standard potential of zero, and all other reduction half-reactions are measured relative to this zero point. Substances less easily reduc ...
Complete Solution Manual
... The zero point for standard reduction potentials (E) is the standard hydrogen electrode. The half-reaction is: 2 H+ + 2 e → H2. This half-reaction is assigned a standard potential of zero, and all other reduction half-reactions are measured relative to this zero point. Substances less easily reduc ...
... The zero point for standard reduction potentials (E) is the standard hydrogen electrode. The half-reaction is: 2 H+ + 2 e → H2. This half-reaction is assigned a standard potential of zero, and all other reduction half-reactions are measured relative to this zero point. Substances less easily reduc ...
mod-5-revision-guide-4-transition-metals
... The initial uncatalysed reaction is slow because the reaction is a collision between two negative ions which repel each other leading to a high activation energy. The Mn2+ ions produced act as an autocatalyst and therefore the reaction starts to speed up because they bring about the alternative reac ...
... The initial uncatalysed reaction is slow because the reaction is a collision between two negative ions which repel each other leading to a high activation energy. The Mn2+ ions produced act as an autocatalyst and therefore the reaction starts to speed up because they bring about the alternative reac ...