Powerpoints - Holy Cross Collegiate
... • Stoichiometry calculations can be used to predict the maximum quantity of product expected from a reaction. This quantity is known as the predicted yield (which is also known as the theoretical yield). • The predicted yield is calculated on the assumption that all the limiting reactant reacts to m ...
... • Stoichiometry calculations can be used to predict the maximum quantity of product expected from a reaction. This quantity is known as the predicted yield (which is also known as the theoretical yield). • The predicted yield is calculated on the assumption that all the limiting reactant reacts to m ...
Chemistry
... A range of reactions, including displacement reactions of metals, combustion, corrosion, and electrochemical processes, can be modelled as redox reactions involving oxidation of one substance and reduction of another substance o ...
... A range of reactions, including displacement reactions of metals, combustion, corrosion, and electrochemical processes, can be modelled as redox reactions involving oxidation of one substance and reduction of another substance o ...
Chemistry Fall Final Study Guide Concepts
... A scale or balance measures mass. A graduated cylinder measures the volume of liquids. A Bunsen burner and hot plate heat substances. 4. What would you observe for H2O(s), H2O(l), H2O(g), and NaCl (aq)? I would observe ice, water, steam (water vapor), and salt water (salt dissolved in water). 5. Usi ...
... A scale or balance measures mass. A graduated cylinder measures the volume of liquids. A Bunsen burner and hot plate heat substances. 4. What would you observe for H2O(s), H2O(l), H2O(g), and NaCl (aq)? I would observe ice, water, steam (water vapor), and salt water (salt dissolved in water). 5. Usi ...
The Law of Definite Proportions
... “A given compound always contains elements in a certain proportion by mass” The ratios by mass of the elements in that compound are fixed independent of the origins or preparation of that compound. A compound is unique because of the specific arrangement and weights of the elements which make up ...
... “A given compound always contains elements in a certain proportion by mass” The ratios by mass of the elements in that compound are fixed independent of the origins or preparation of that compound. A compound is unique because of the specific arrangement and weights of the elements which make up ...
Chapter 2: Matter Is Made up of Atoms
... Politics and Chemistry—Elemental Differences In the time of Antoine Lavoisier (1743-1794), many scientists were still trying to explain matter as combinations of the elements air, earth, fire, and water. Lavoisier’s work changed the way chemistry was done, and today he is recognized as the first mod ...
... Politics and Chemistry—Elemental Differences In the time of Antoine Lavoisier (1743-1794), many scientists were still trying to explain matter as combinations of the elements air, earth, fire, and water. Lavoisier’s work changed the way chemistry was done, and today he is recognized as the first mod ...
Electrons in atoms
... • The aufbau diagram can be used to write correct ground-state electron configurations for all elements up to and including Vanadium, atomic number 23. • The electron configurations for certain transition metals, like chromium and copper, do not follow the aufbau diagram due to increased stability o ...
... • The aufbau diagram can be used to write correct ground-state electron configurations for all elements up to and including Vanadium, atomic number 23. • The electron configurations for certain transition metals, like chromium and copper, do not follow the aufbau diagram due to increased stability o ...
The Copper Cycle
... In Part V, zinc metal (Zn) is added to the copper solution to convert the copper ions back to copper metal, Cu(s). The resulting solution will contain colorless zinc ions, Zn2+(aq) and copper solid. Visible evidence of this reaction is observed as bubbles of gas being released from the solution. (Si ...
... In Part V, zinc metal (Zn) is added to the copper solution to convert the copper ions back to copper metal, Cu(s). The resulting solution will contain colorless zinc ions, Zn2+(aq) and copper solid. Visible evidence of this reaction is observed as bubbles of gas being released from the solution. (Si ...
Atomic Structure Practice Test
... REF: 1 OBJ: 3 STA: SC.B.1.4.2 26. ANS: The atomic number equals the number of protons in the nucleus of an atom and also equals the number of electrons in the neutral atom. The mass number is the sum of the number of protons and neutrons and can be used, with the atomic number, to find the number of ...
... REF: 1 OBJ: 3 STA: SC.B.1.4.2 26. ANS: The atomic number equals the number of protons in the nucleus of an atom and also equals the number of electrons in the neutral atom. The mass number is the sum of the number of protons and neutrons and can be used, with the atomic number, to find the number of ...
Molecular Formulas - Hatboro
... number of neutrons), X is the element’s symbol, and Z is the atomic number (number of protons). This information can also be found on the periodic table. Use this information to fill in the chart for the following elements Symbol ...
... number of neutrons), X is the element’s symbol, and Z is the atomic number (number of protons). This information can also be found on the periodic table. Use this information to fill in the chart for the following elements Symbol ...
physical setting chemistry
... Setting/Chemistry, and your knowledge of chemistry. In the 1920s, paint used to inscribe the numbers on watch dials was composed of a luminescent (glow-in-the-dark) mixture. The powdered-paint base was a mixture of radium salts and zinc sulfide. As the paint was mixed, the powdered base became airbo ...
... Setting/Chemistry, and your knowledge of chemistry. In the 1920s, paint used to inscribe the numbers on watch dials was composed of a luminescent (glow-in-the-dark) mixture. The powdered-paint base was a mixture of radium salts and zinc sulfide. As the paint was mixed, the powdered base became airbo ...
Boundless Study Slides
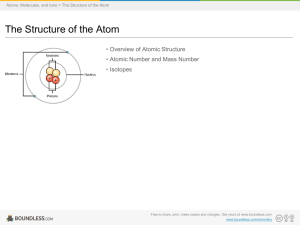
... • An atom is composed of two regions: the nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom which holds its electrons in orbit around the nucleus. • Protons and neutrons have approximately the same mass, about 1.67 × 10-24 grams, which sc ...
... • An atom is composed of two regions: the nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom which holds its electrons in orbit around the nucleus. • Protons and neutrons have approximately the same mass, about 1.67 × 10-24 grams, which sc ...
chemistry 2.1
... • how much is present in a chemical substance, or • how much is involved in a chemical reaction, for one or more chemicals. Significant figures indicate the level of accuracy of the data and/or apparatus. In calculations, final answers typically include three significant figures, and no rounding sho ...
... • how much is present in a chemical substance, or • how much is involved in a chemical reaction, for one or more chemicals. Significant figures indicate the level of accuracy of the data and/or apparatus. In calculations, final answers typically include three significant figures, and no rounding sho ...
1 Introduction to Atoms
... Figuring out what atoms are made of hasn’t been easy. Theories about their shape and structure have changed many times and continue to be improved even now. Until about 100 years ago, scientists thought atoms were the smallest particles of matter. Now, scientists know more. Atoms are made of even sm ...
... Figuring out what atoms are made of hasn’t been easy. Theories about their shape and structure have changed many times and continue to be improved even now. Until about 100 years ago, scientists thought atoms were the smallest particles of matter. Now, scientists know more. Atoms are made of even sm ...
Slide 1
... Around 492-432 BC, the Greek Empedocle divided matter into four elements, called "roots": earth, air, fire and water Elements like gold, silver, tin, copper, lead, and mercury have been known since ancient times Mendeleev’s periodic table (1869) ...
... Around 492-432 BC, the Greek Empedocle divided matter into four elements, called "roots": earth, air, fire and water Elements like gold, silver, tin, copper, lead, and mercury have been known since ancient times Mendeleev’s periodic table (1869) ...
Chapter 1 - TamAPChemistryHart
... a) The nucleus has most of the mass and comprises most of the volume of an atom; b) Every atom of a given element has the same number of protons; c) The number of electrons in an atom equals the number of neutrons in the atom; d) The protons in the nucleus of the helium atom are held together by a f ...
... a) The nucleus has most of the mass and comprises most of the volume of an atom; b) Every atom of a given element has the same number of protons; c) The number of electrons in an atom equals the number of neutrons in the atom; d) The protons in the nucleus of the helium atom are held together by a f ...
AP Chemistry - Forsyth County Schools
... Blank periodic table (here is a link, or you can search/use one of your own): http://www.csudh.edu/oliver/chemdata/periodic/periodic-1.htm A review book is highly recommended, though not required. Princeton Review, and/or 5 steps to a 5 are all good for different reasons. Barrons is also an opti ...
... Blank periodic table (here is a link, or you can search/use one of your own): http://www.csudh.edu/oliver/chemdata/periodic/periodic-1.htm A review book is highly recommended, though not required. Princeton Review, and/or 5 steps to a 5 are all good for different reasons. Barrons is also an opti ...
Worksheet to accompany demos on exchange reactions
... atom must get short-shrifted! That is, the electrons ―assigned‖ to O were effectively ―taken away‖ from C. Since the two O atoms each get 2 extra electrons, the C atom must be deficient by 4 electrons, and so it would have 4 fewer electrons than a neutral C atom and so it would have a ―fictitious‖ c ...
... atom must get short-shrifted! That is, the electrons ―assigned‖ to O were effectively ―taken away‖ from C. Since the two O atoms each get 2 extra electrons, the C atom must be deficient by 4 electrons, and so it would have 4 fewer electrons than a neutral C atom and so it would have a ―fictitious‖ c ...
The Nuclear Model of the Atom
... 27. The particles used in Rutherford’s experiment were about _____ times the size of the hydrogen atom and were ___________ charged. 28. Rutherford’s atomic model became known as the nuclear model. In this model, the protons and neutrons, which comprise nearly all of the mass of the atom, are locate ...
... 27. The particles used in Rutherford’s experiment were about _____ times the size of the hydrogen atom and were ___________ charged. 28. Rutherford’s atomic model became known as the nuclear model. In this model, the protons and neutrons, which comprise nearly all of the mass of the atom, are locate ...
Modern Physics
... Rutherford assumed the electrons must be in some kind of orbits around the nucleus that extended out to the size of the atom. Major problem is that electrons would be undergoing centripetal acceleration and should emit EM waves, lose energy and spiral into the nucleus! Not very satisfactory situatio ...
... Rutherford assumed the electrons must be in some kind of orbits around the nucleus that extended out to the size of the atom. Major problem is that electrons would be undergoing centripetal acceleration and should emit EM waves, lose energy and spiral into the nucleus! Not very satisfactory situatio ...
Unit 3, Lesson 07: Calculating ∆H using Standard Enthalpies of
... 2. Hess’s Law when you know ∆H values for other chemical reactions that can be added to give you your target chemical reaction 3. Standard Molar Enthalpies of Formation (∆Hºf) • defined as the amount of energy released or absorbed when one mole of a compound is formed directly from its elements, in ...
... 2. Hess’s Law when you know ∆H values for other chemical reactions that can be added to give you your target chemical reaction 3. Standard Molar Enthalpies of Formation (∆Hºf) • defined as the amount of energy released or absorbed when one mole of a compound is formed directly from its elements, in ...
lectures on subjects in physics, chemistry and biology
... pressure it is found that the current is carried by electrons moving in one direction and positively charged atoms moving in the opposite direction. T h e stream of positively charged atoms can be allowed to pass through a hole in the negative electrode and so may be separated from the stream of ele ...
... pressure it is found that the current is carried by electrons moving in one direction and positively charged atoms moving in the opposite direction. T h e stream of positively charged atoms can be allowed to pass through a hole in the negative electrode and so may be separated from the stream of ele ...
Powerpoints for Chapter 4
... The Hydrogen-Atom Line-Emission Spectrum, continued • When investigators passed electric current through a vacuum tube containing hydrogen gas at low pressure, they observed the emission of a characteristic pinkish glow. • When a narrow beam of the emitted light was shined through a prism, it was se ...
... The Hydrogen-Atom Line-Emission Spectrum, continued • When investigators passed electric current through a vacuum tube containing hydrogen gas at low pressure, they observed the emission of a characteristic pinkish glow. • When a narrow beam of the emitted light was shined through a prism, it was se ...